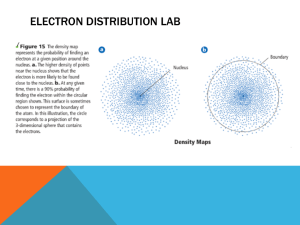
Atomic Structure
advertisement

Bell Work 1. PRE-AP ONLY: Draw an Aufbau Chart. 2. Write the Electron Configuration for 1. 2. 3. 4. H LI P Fe 3. What does the Aufbau Principle say? What You Should Know How to Do 1. Aufbau Chart: 1. Pre-AP Draw & Read 2. Chem I Read 2. Write Electron Configurations 3. State Aufbau Principle Spherical Radii of the Orbitals Pauli Exclusion Principle • Electrons are subject to the Pauli Exclusion Principle, so: No electron can occupy the same quantum state as another • So, how do 2 electrons occupy the same energy level and orbital? Pauli Exclusion Principle • They have “spin” • electrons in the same orbital will have different “spins” – An analogy is to think of it as clockwise and counterclockwise spinning • These spins are called “up” spin and “down” spin – They are written as Hund’s Rule • Each sub-orbital will have 1 electron before any get a second • Two ways to think of it 1. The Candy Rule – it’s not fair if some get 2 pieces of candy and some get none, first everyone gets a piece, then we start handing out a second piece 2. The Urinal Rule Hund’s Rule • Two ways to think of it 1. The Candy Rule – it’s not fair if some get 2 pieces of candy and some get none, first everyone gets a piece, then we start handing out a second piece 2. The Urinal Rule (for the guys) – no “pairing” until you have to Which is Correct? Class Work 1. Show Hund’s Rule for: 1. 2. 3. 4. H He C F 2. Write the electron configuration for H, N, Na, Cl, K, V, Cu, Se, Kr, Y Exit Ticket • Draw Hund’s Rule for: – Li – Ne • Write the electron configuration for: –B – Al – Ga