Aufbau Diagram Directions
advertisement
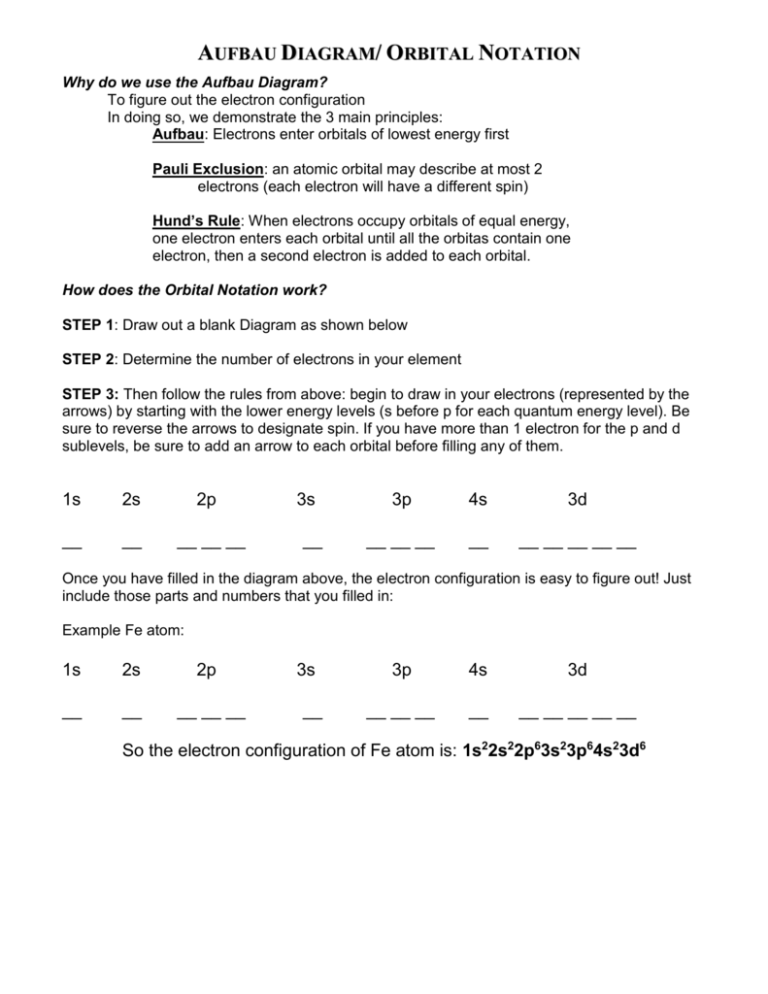
AUFBAU DIAGRAM/ ORBITAL NOTATION Why do we use the Aufbau Diagram? DIRECTIONS To figure out the electron configuration In doing so, we demonstrate the 3 main principles: Aufbau: Electrons enter orbitals of lowest energy first Pauli Exclusion: an atomic orbital may describe at most 2 electrons (each electron will have a different spin) Hund’s Rule: When electrons occupy orbitals of equal energy, one electron enters each orbital until all the orbitas contain one electron, then a second electron is added to each orbital. How does the Orbital Notation work? STEP 1: Draw out a blank Diagram as shown below STEP 2: Determine the number of electrons in your element STEP 3: Then follow the rules from above: begin to draw in your electrons (represented by the arrows) by starting with the lower energy levels (s before p for each quantum energy level). Be sure to reverse the arrows to designate spin. If you have more than 1 electron for the p and d sublevels, be sure to add an arrow to each orbital before filling any of them. 1s 2s 2p __ __ __ __ __ 3s __ 3p 4s 3d __ __ __ __ __ __ __ __ __ Once you have filled in the diagram above, the electron configuration is easy to figure out! Just include those parts and numbers that you filled in: Example Fe atom: 1s 2s 2p __ __ __ __ __ 3s __ 3p 4s 3d __ __ __ __ __ __ __ __ __ So the electron configuration of Fe atom is: 1s22s22p63s23p64s23d6