Mg P V Ge - EricksonCPChem2010-11
advertisement
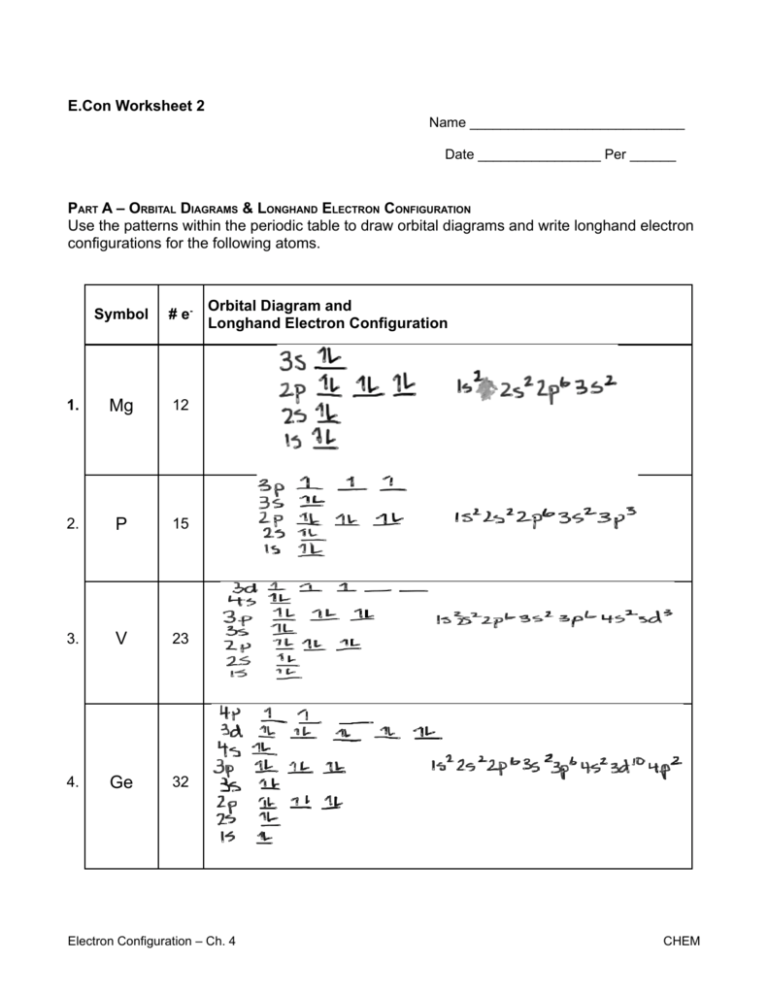
E.Con Worksheet 2 Name ____________________________ Date ________________ Per ______ PART A – ORBITAL DIAGRAMS & LONGHAND ELECTRON CONFIGURATION Use the patterns within the periodic table to draw orbital diagrams and write longhand electron configurations for the following atoms. Symbol # e- 1. Mg 12 2. P 15 3. V 23 4. Ge 32 Orbital Diagram and Longhand Electron Configuration Electron Configuration – Ch. 4 CHEM 5. Kr 36 6. O 8 Electron Configuration – Ch. 4 CHEM PART B – SHORTHAND ELECTRON CONFIGURATION Use the patterns within the periodic table to write the shorthand electron configurations for the following elements. Symbol 7. Ca 8. Ba # e20 Shorthand Electron Configuration Not required 9 9. F 1 0. Ba 56 PART B – RULES OF ELECTRON CONFIGURATIONS Which of the following “rules” is being violated in each electron configuration below? Explain your answer for each. Hund’s Rule, Pauli Exclusion Principle, Aufbau Principle 1 1. 1s __ __ 2s 2p Hund's Rule – every orbital must be singly occupied first 1 2. 1s ___ _ _ 2s 2p 3s 3p Aufbau Principle – lowest energy orbital occupied first (ie. 3S before 3p) 1 3. 1s _ 2s 2p 3s 3p Pauli Exclusion Principle – 3s orbital cannot have electrons with same spin direction. Stability & Electron Configuration – Ch. 4 CHEM 1 1s 4. 2s 2p 3s 3p 3d Aufbau Principle – occupy lowest energy orbitals first and 4s comes before 3d Stability & Electron Configuration – Ch. 4 CHEM






