H midyear review ch 7
Logged in as tkoby@whr.nj.
WebAssign.net
Logout
Sunday, January 16, 2005 06:52 PM EST
Home | My Assignments | Grades | Communication | Calendar
Home > My Assignments > H midyear
review ch 7 (Homework)
Guide | Help | My Options
Timothy Koby
ChemistryH, section Period 11, 20042005
Instructor: Victoria Hubinger
Watchung Hills Regional High School
About this Assignment
Due: Monday, January 24, 2005 07:26 AM
EST
Current Score: 69 out of 69
Question Score
pts subs
1 1/1 2/15
2 1/1 1/15
2/2
Viewing:
Last
Response
View:
All
Responses
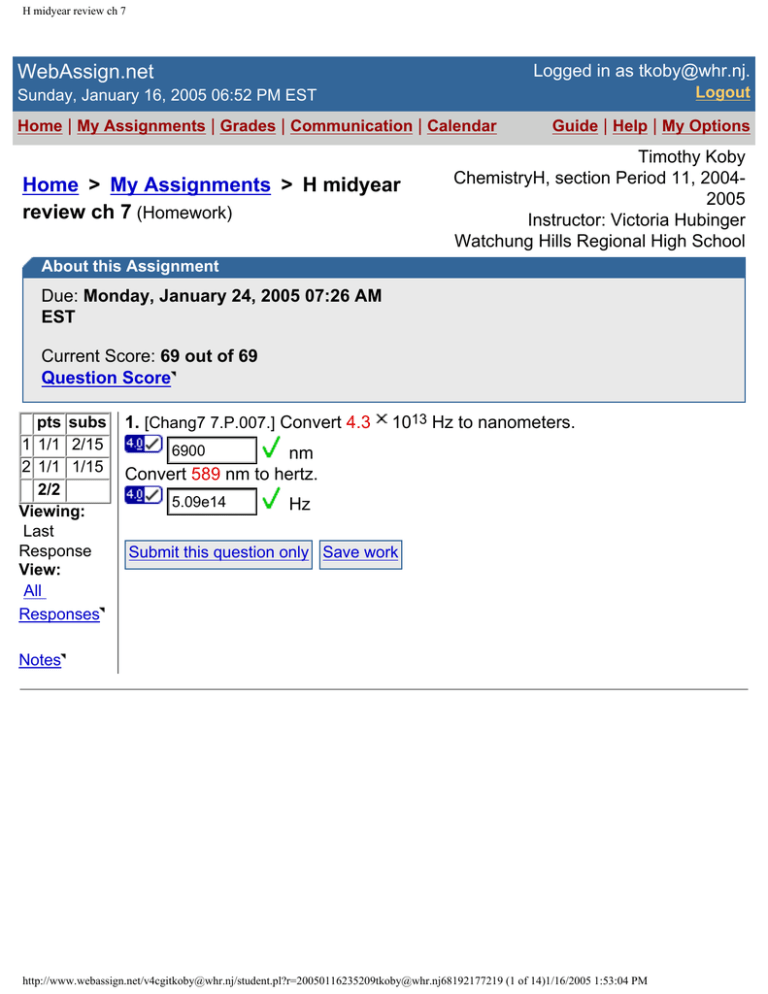
1. [Chang7 7.P.007.] Convert 4.3
1013 Hz to nanometers.
6900
nm
Convert 589 nm to hertz.
5.09e14
Hz
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (1 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
pts subs
1 1/1 1/15
2 1/1 4/15
2/2
Viewing:
Last
Response
View:
All
Responses
2. [Chang7 7.P.008.] (a) What is the frequency of light having a wavelength of
403 nm?
7.44e14
Hz
(b) What is the wavelength (in nanometers) of radiation having a frequency of
2.31 109 Hz? (This is the type of radiation used in microwave ovens.)
129000000
nm
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 3/15
4 1/1 1/15
4/4
Viewing:
Last
Response
View:
All
Responses
Notes
3. [Chang7 7.P.017.] A photon has a frequency of 5.6
104 Hz.
(a) Convert this frequency into wavelength (nm).
5.4e12
nm
Does this frequency fall in the visible region?
yes
no
(b) Calculate the energy of this photon.
3.7e-29
J
(c) Calculate the energy of one mole of photons all with this
frequency.
2.2e-5
J
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (2 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
pts subs
1 1/1 5/15
2 1/1 2/15
3 1/1 1/15
3/3
Viewing:
Last
Response
View:
All
Responses
Notes
4. [Chang7 7.P.029.] Consider the following energy levels of a hypothetical atom.
E4
-1.0
10-19 J
E3
-5.0
10-19 J
E2
-10
10-19 J
E1
-15
10-19 J
(a) What is the wavelength of the photon needed to excite an
electron from E1 to E4?
140
nm
(b) What is the energy (in joules) a photon must have in order to
excite an electron from E2 to E3?
5e-19
J
(c) When an electron drops from the E3 level to the E1 level, the
atom is said to undergo emission. Calculate the wavelength of the
photon emitted in this process.
199
nm
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (3 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
3/3
Viewing:
Last
Response
View:
All
Responses
5. [Chang7 7.P.055.] Give the values of the quantum numbers associated with
the following orbitals.
(a) 2p
m = only 0
l
m = only 1
l
m = 1, 0, or -1
l
(b) 3s
m =1
l
Notes
m = -1
l
m =0
l
(c) 5d
m = only -2
l
m = only 2
l
m = 2, 1, 0, -1, or -2
l
Submit this question only Save work
pts subs
1 1/1 2/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
4/4
Viewing:
Last
Response
View:
All
Responses
6. [Chang7 7.P.061.] Calculate the total number of electrons that can occupy the
following orbitals.
(a) one s orbital
one
two
three
six
(b) three p orbitals
three
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (4 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
six
nine
twelve
Notes
(c) five d orbitals
five
nine
ten
fifteen
(d) seven f orbitals
six
seven
twelve
fourteen
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 3/15
5 1/1 1/15
6 1/1 1/15
6/6
Viewing:
Last
Response
View:
All
Responses
7. [Chang7 7.P.084.] Write the ground-state electron configurations for the
following elements. (Type your answer in noble gas notation using the format
[Ar] 4s2 3d10 4p2 for [Ar]4s23d104p2).
Zn
[Ar]4s2 3d10
Sb
[Kr] 5s2 4d10 5p3
Rb
[Kr] 5s1
W
[Xe] 6s2 4f14 5d4
V
[Ar] 4s2 3d3
Notes
Ni
[Ar] 4s2 3d8
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (5 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
Submit this question only Save work
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
8. [Chang7 7.P.091.] Use the Aufbau principle to obtain the ground-state electron
configuration of silicon. (Type your answer in noble gas notation using the
format [Ar] 4s2 3d10 4p2 for [Ar]4s23d104p2.)
[Ne] 3s2 3p2
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
5 1/1 1/15
6 1/1 1/15
7 1/1 4/15
8 1/1 1/15
9 1/1 3/15
10 1/1 2/15
10/10
Viewing:
Last
Response
View:
All
Responses
Notes
9. [Chang7 7.P.114.] The electron configurations described in this chapter all
refer to gaseous atoms in their ground states. An atom may absorb a
quantum of energy and promote one of its electrons to a higher-energy
orbital. When this happens, we say that the atom is in an excited state. The
electron configurations of some excited atoms are given. Identify these
atoms and write their ground-state configurations. (Type your answer using
the format [Ar] 4s2 3d10 4p2 for [Ar]4s23d104p2.)
(a) 1s12s1
name
Helium
ground state configuration
1s2
(b) 1s22s22p23d1
name
Nitrogen
ground state configuration
1s2 2s2 2p3
(c) 1s22s22p64s1
name
Sodium
ground state configuration
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (6 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
1s2 2s2 2p6 3s1
(d) [Ar]4s13d104p4
name
Arsenic
ground state configuration
[Ar] 4s2 3d10 4p3
(e) [Ne]3s23p43d1
name
Chlorine
ground state configuration
[Ne] 3s2 3p5
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
Notes
10. [Chang7 7.P.120.] An electron in a hydrogen atom is excited from the ground
state to the n = 4 state. Comment on the correctness of the following
statements.
(a) n = 4 is the first excited state.
true
false
(b) It takes more energy to ionize (remove) the electron from n = 4
than from the ground state.
true
false
(c) The electron is farther from the nucleus (on average) in n = 4
than in the ground state.
true
false
(d) The wavelength of light emitted when the electron drops from n
= 4 to n = 1 is longer than that from n = 4 to
n = 2.
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (7 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
true
false
(e) The wavelength the atom absorbs in going from n = 1 to n = 4
is the same as that emitted as it goes from n = 4 to n = 1.
true
false
Submit this question only Save work
pts subs
1 1/1 3/15
2 1/1 1/15
3 1/1 2/15
4 1/1 1/15
5 1/1 1/15
6 1/1 1/15
6/6
Viewing:
Last
Response
View:
All
Responses
11. [Chang7 7.P.124.] Shown below are portions of orbital diagrams representing
the ground-state electron configurations of certain elements. Which of them
violate the Pauli exclusion principle? Which of them violate Hund's rule?
(a)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
(b)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
Notes
(c)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (8 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
(d)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
(e)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
(f)
violates the Pauli exclusion principle
violates the Hund's rule
violates both Pauli exclusion principle and Hund's rule
does not violate Pauli exclusion principle or Hund's rule
Submit this question only Save work
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
Notes
12. [Chang7 7.TB.029b.] Consider the following energy levels of a hypothetical
atom.
E4 = -1.0
10-19 J
E3 = -5.0
10-19 J
E2 = -10
10-19 J
E1 = -15
10-19 J
What is the energy (in joules) a photon must have in order to excite an electron
from E2 to E3?
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (9 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
5 10-10 J
10 10-19 J
15 10-19 J
5 10-19 J
Submit this question only Save work
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
13. [Chang7 7.TB.054.] An electron in an atom is in the n = 3 quantum level. List
the possible values of l and ml that it can have.
l = 0, ml = 0; l = 1, ml = -1, 0, 1; l = 2, ml = -2, -1, 0, 1, 2
l = 0, ml = 0; l = 1, ml = 0, 1; l = 2, ml = 0, 1, 2
l = 0, ml = 0; l = 1, ml = -1, 0, 1; l = 2, ml = -2, -1, 0, 1, 2; l = 3, ml = -3,
-2, -1, 0, 1, 2, 3
l = 1, ml = -1, 0, 1; l = 2, ml = -2, -1, 0, 1, 2
Notes
Submit this question only Save work
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
14. [Chang7 7.TB.055c.] Give the values of the quantum numbers associated
with the 5d orbital.
n = 5, l = 1, ml = -1, 0, 1
Notes
Submit this question only Save work
n = 5, l = 0, ml = 0
n = 5, l = 3, ml = -3, -2, -1, 0, 1, 2, 3
n = 5, l = 2, ml = -2, -1, 0, 1, 2
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (10 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
15. [Chang7 7.TB.056a.] State whether the values of the quantum numbers (n, l,
and ml) and the number of orbitals in the subshell are true or false.
4p subshell: n = 4, l = 3, ml = -3, -2, -1, 0, 1, 2, 3; 3 p orbitals.
true
false
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
16. [Chang7 7.TB.056d.] State whether the values of the quantum numbers (n, l,
and ml) and the number of orbitals in the subshell are true or false.
5f subshell: n = 5, l = 3, ml = 0, 1, 2, 3; 7f orbitals.
true
false
Submit this question only Save work
Notes
pts subs
1 1/1 3/15
2 1/1 1/15
3 1/1 1/15
4 1/1 3/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
Notes
17. [Chang7 7.TB.067.] For each of the following pairs of hydrogen orbitals,
indicate which is higher in energy.
(a) 1s, 2s
1s
2s
equal
(b) 2p, 3p
2p
3p
equal
(c) 3dxy, 3dyz
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (11 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
3dxy
3dyz
equal
(d) 3s, 3d
3s
3d
equal
(e) 4f, 5s
4f
5s
equal
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 2/15
4 1/1 1/15
5 1/1 2/15
6 1/1 2/15
6/6
Viewing:
Last
Response
View:
All
Responses
18. [Chang7 7.TB.082a.] Indicate the number of unpaired electrons present in
each of the following atoms.
(a) B
1
(b)Ne
0
(c) P
3
(d) Sc
1
(e) Mn
5
Notes
(f) Se
2
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (12 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
19. [Chang7 7.TB.091.] Use the Aufbau principle to obtain the ground-state
electron configuration of selenium.
Se: [Ar]4s23d104p6
Se: [Ar]4s23d104p3
Se: [Ar]4s23d104p4
Se: [Ar]4s23d104p5
Submit this question only Save work
Notes
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
20. [Chang7 7.TB.093.] When a compound containing cesium ion is heated in a
Bunsen burner flame, photons with an energy of 4.30 10-19 J are emitted.
What color is the cesium flame?
Violet
Yellow
Blue
Green
Notes
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 2/15
3 1/1 1/15
4 1/1 1/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
21. [Chang7 7.TB.096.] What is the maximum number of electrons in an atom
that can have the following quantum numbers?
(a) n = 2, ms = +1/2
4
(b) n = 4, ml = +1
6
(c) n = 3, l = 2
10
(d) n = 2, l = 0, ms = -1/2
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (13 of 14)1/16/2005 1:53:04 PM
H midyear review ch 7
1
Notes
(e) n = 4, l = 3, ml = -2
2
Submit this question only Save work
Submit all questions for grading
Save all work
Home My Assignments
WebAssigntm 4.0 © 1997-2005 by North Carolina State University. All rights reserved.
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?r=20050116235209tkoby@whr.nj68192177219 (14 of 14)1/16/2005 1:53:04 PM