Electron Configurations
advertisement
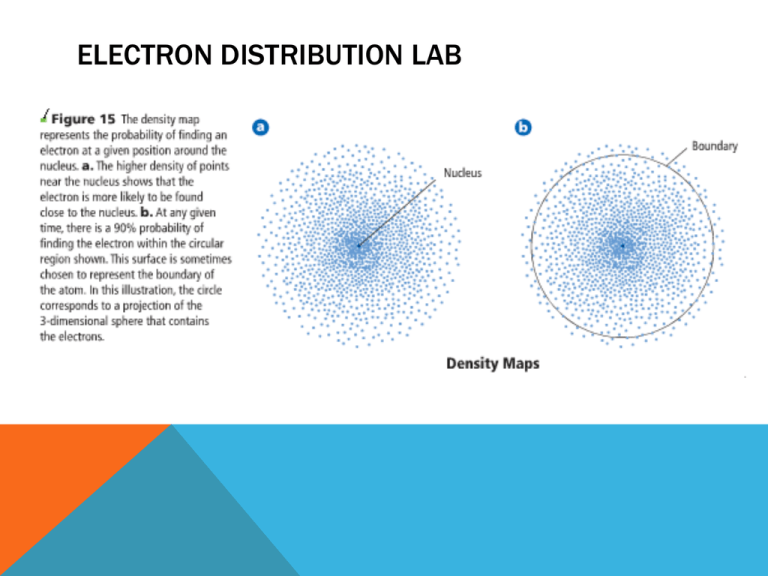
ELECTRON DISTRIBUTION LAB WHAT IS AN ELECTRON CONFIGURATION? The arrangement of electrons in an atom THREE RULES TO REMEMBER… • 1. Aufbau Principle • 2. Pauli Exclusion Principle • 3. Hund’s Rule AUFBAU PRINCIPLE • States that each electron occupies the lowest energy orbital available • Must learn the sequence of atomic orbitals from lowest energy to highest energy • Called the Aufbau diagram! • Each box represents an atomic orbital HOW TO REMEMBER THE AUFBAU PRINCIPLE • Principal quantum numbers are like floors of a hotel • e- enters the lowest level first PAULI EXCLUSION PRINCIPLE • Electrons in orbitals can be represented by arrows in boxes. • Every electron has an associated spin • Can only spin two ways Represents electron spinning in one direction Represents electron spinning in opposite direction Represents unoccupied orbital Represents a filled orbital Pauli Exclusion Principle States that a maximum of TWO electrons can occupy a single atomic orbital, but only if they have opposite spins. HOW TO REMEMBER THE PAULI EXCLUSION PRINCIPLE • Spin one index finger away from you • Spin the other index finger toward you HUND’S RULE • Single electrons with the same spin must occupy each equal energy orbital before additional electrons with opposite spins can occupy the same orbital. How to Remember…. Strangers on a Bus! Do you sit next to a stranger… or pick an empty seat? Pick an empty seat, unless there isn’t one, then you sit with the stranger… Orbital Diagram for Phosphorus ORBITAL DIAGRAMS CONT. • Draw the orbital diagram for • Sodium • Chlorine ORBITAL & ELECTRON CONFIGURATION NOTATION ELECTRON CONFIGURATION NOTATION 1s Silicon 2s 2p 3s 3p 3d 4s 4p 4d 5f 5s 5p 5d 5f 6s 6p 6d 7s 7f Remember • • • • 1 s-orbital 3 p-orbitals 5 d-orbitals 7 f-orbitals




![6) cobalt [Ar] 4s 2 3d 7](http://s2.studylib.net/store/data/009918562_1-1950b3428f2f6bf78209e86f923b4abf-300x300.png)