8th Science Fall Midterm Review Sheet Understand the structure of
advertisement

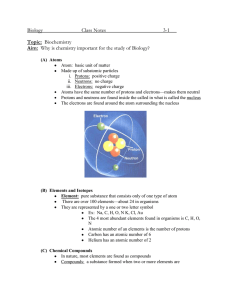
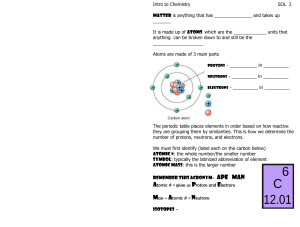
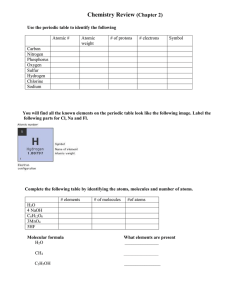
8th Science Fall Midterm Review Sheet Understand the structure of atoms, including masses, electrical charges, and locations of protons, neutrons, and electrons. To find the number of neutrons in an atom, subtract the atomic mass (rounded to the nearest whole number) minus the atomic number (number of protons). Atomic mass= atomic number (protons) + neutrons Know that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity. Know the difference between and be able to identify elements and compounds. Be able to compare metals, nonmetals and metalloids. Identify that all organic compounds contain carbon. Interpret the arrangement of the Periodic Table. G R O U Period Know that elements within the same group (family) have similar properties. Know the number of valence electrons for groups 1, 2, and 13-18. Be able to determine the number of atoms of each element in chemical formulas containing subscripts. Know the evidences of a chemical reaction. Ex.-color change, bubbles or fizzing, precipitate is formed, new substance is formed, smoke or fire, and unexpected temperature change. The only sure way to know if a chemical change occurred is if a new substance was formed. Recognize whether a chemical equation containing coefficients is balanced or not and how that relates to the law of conservation of mass. The law of conservation of mass states that during a chemical reaction, matter is not created or destroyed