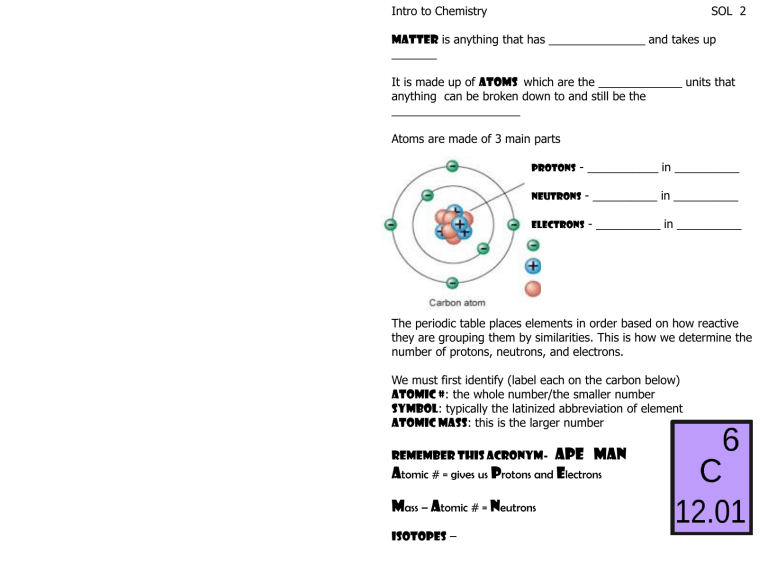
Intro to Chemistry SOL 2 Matter is anything that has _______________ and takes up _______ It is made up of atoms which are the _____________ units that anything can be broken down to and still be the ____________________ Atoms are made of 3 main parts Protons - ___________ in __________ Neutrons Electrons - __________ in __________ - __________ in __________ The periodic table places elements in order based on how reactive they are grouping them by similarities. This is how we determine the number of protons, neutrons, and electrons. We must first identify (label each on the carbon below) Atomic #: the whole number/the smaller number Symbol: typically the latinized abbreviation of element Atomic Mass: this is the larger number APE MAN Atomic # = gives us Protons and Electrons Remember this Acronym- Mass – Atomic # = Neutrons Isotopes – Element: composed of a ____________________ Ex: Lithium Sulfur Compound: two or more ____________ atoms chemically bonded together Ex: Molecule: two or more of the ____________ or ______________ elements chemically bonded together Ex. Mixture: two or more substances that are __________ chemically bonded together Ex: Drawing an element The 1st shell holds The 2nd shell holds The 3rd shell holds For this class we will stick with _____ in the next orbit as well. Ionic Bond Sodium Chlorine Carbon: How many electrons How many protons How many neutrons Try oxygen: Hydrogen Electrons= Protons= Neutrons= Covalent Bond Oxygen Hydrogen



