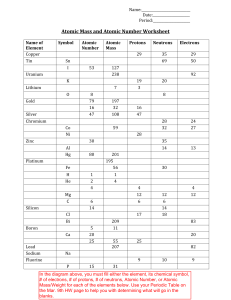
SubatomicParticlePractice
advertisement
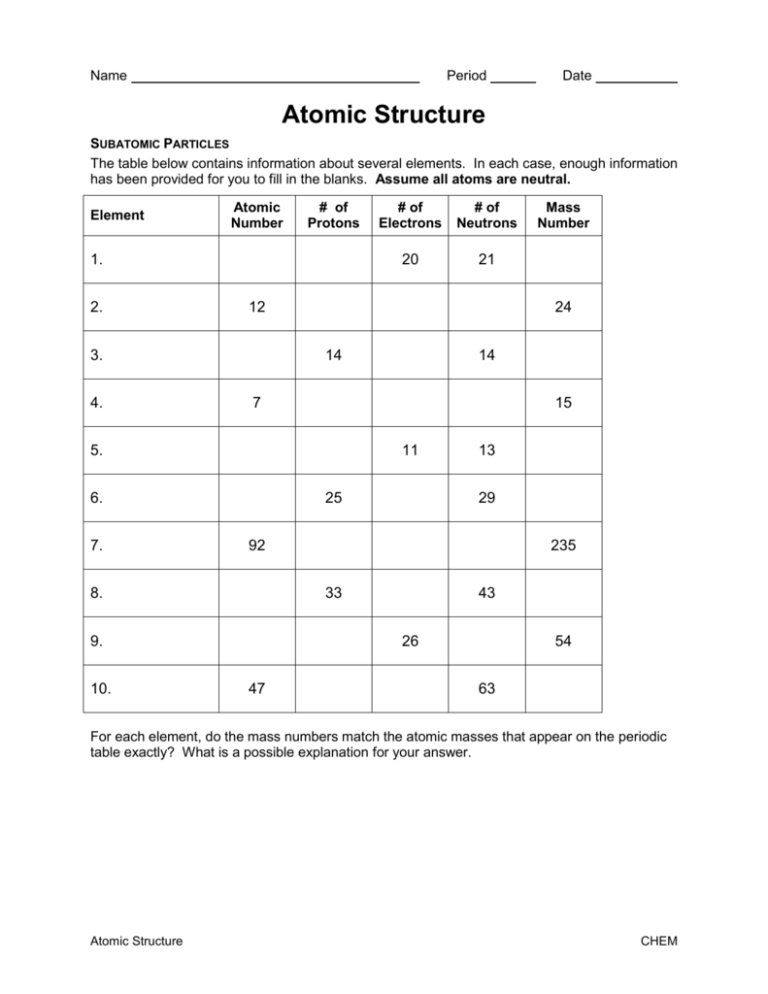
Name Period Date Atomic Structure SUBATOMIC PARTICLES The table below contains information about several elements. In each case, enough information has been provided for you to fill in the blanks. Assume all atoms are neutral. Element Atomic Number # of Protons 1. 2. 21 14 14 7 15 11 6. 25 13 29 92 8. 235 33 9. 10. 20 43 26 47 Mass Number 24 5. 7. # of Neutrons 12 3. 4. # of Electrons 54 63 For each element, do the mass numbers match the atomic masses that appear on the periodic table exactly? What is a possible explanation for your answer. Atomic Structure CHEM