Chemistry Worksheet: Atomic Structure & Isotopes
advertisement

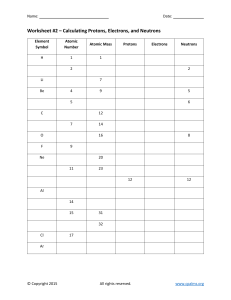
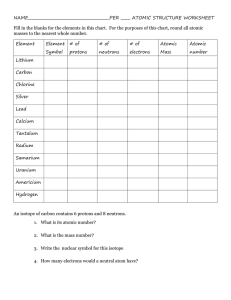
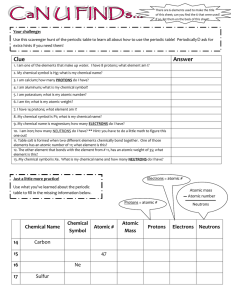
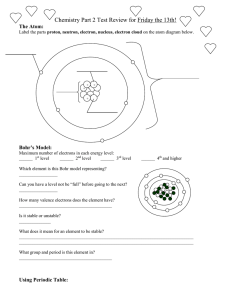
UNIT 2 REVIEW Concepts: Dalton’s atomic theory, chemical formula, parts of an atom (proton, electron, neutron), nucleus, isotopes, atomic #, mass #, ion, diatomic molecule, ionic compound, periodic table 1. Draw the atom symbol for Europium with a mass number of 155. 2. How many protons do each of the following atoms have (a little element practice while you’re at it)? Tungsten ____ Ra_____ Manganese _____ Tin_____ Iron____ Br _____ S____ Barium ____ 3. Fill out the table with all of the missing information: Symbol Atomic number Mass number 79 185 Protons Neutrons Electrons V 49 79 18 24 18 230 90 92 100 44 41 Pb4+ 210 48 S 31 2- 65 45 4. Using the following symbols, tell how many protons, neutrons and electrons in each: 140 + 94 200 La Which of the above are IONS? Zr +2 Hg Which are ATOMS? How do you know? 5. Complete the following chart: Isotope name atomic # mass # # of protons # of neutrons # of electrons uranium-235 uranium-238 boron-10 boron-11 What do U-235 and U-238 (or B-10 and B-11) have in common? What are they called? 6. Draw an element symbol of a Cesium ion that has 75 neutrons and 56 electrons