Organic Chemistry: Introduction
advertisement

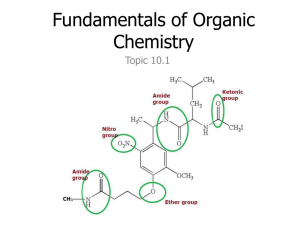
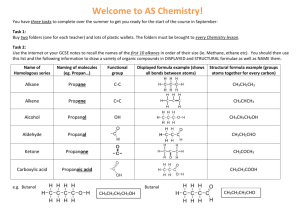
Organic Chemistry Introduction Topic 10.1.9 – 10.1.13 Compounds up to 6 carbon atoms with functional groups (10.1.9) Functional Group Alcohol Formula -OH Aldehyde -COH (on the end Structural Formula -O–H O of a chain) -C–H Ketone -CO- (can’t be on O end of chain) -C– Carboxylic Acid -COOH (on the O end of a chain) -C–O–H Halide -Br, -Cl, -F, -I -X Compounds up to 6 carbon atoms with functional groups (10.1.9-10) Functional Group Formula Alcohol -OH Suffix (or Prefix) -ol Aldehyde -COH -al Ketone -CO- -one Carboxylic Acid -COOH -oic acid Halide -Br, -Cl, -F, -I bromo-,chloro-, fluoro-,iodo- Know these 7, only have to recognize the 3 in the Alcohols: suffix = “ol” 1-propanol or propan-1-ol 2-propanol or propan-2-ol 2-methyl-2-propanol or 2-methyl propan-2-ol Aldehydes: suffix = “al” propanal Note: an aldeyhde group is always on an end carbon so don’t need a number butandianol? Ketones: suffix = “one” propanone (don’t need C#, must be in between two carbons) butanone (don’t need C#, must be in between two carbons) 2-pentanone or penta-2-one butandione pentan-3-one Carboxylic Acids: suffix = “oic acid” butanoic acid Note: a carboxyl is always on an end carbon propandioic acid Halides: prefixes = “fluoro, chloro, bromo, iodo” 1-bromopropane 2-chlorobutane 1,2-diiodoethane 1,2-difluoroethene 1,2-difluoroethene 1,1,2-trifluorothene Only identify the following functional groups in structures: (10.1.11) Functional Formula Group Amine - NH2 Ester Benzene O R–C–O–R amino, benzene ring, ester 10.1.12 Identify primary, secondary and tertiary carbon atoms in alcohols (-OH) and halogenoalkanes (-F, -Cl, -Br, -I) • with reference to the carbon that is directly bonded to an alcohol group or a halogen: – Primary = carbon atom is only bonded to one other carbon – Secondary = carbon atom is bonded to two other carbons – Tertiary = carbon atom is bonded to three other carbons 10.1.13 Discuss the volatility and solubility in water of compounds containing the functional groups listed in10.1.9. • Volatility: how easily a substance turns into a gas – the weaker the intermolecular force, the more volatile it is – van der Wall’s › dipole › hydrogen bonding – alkane › halogenoalkane › aldehyde › ketone › amine › alcohol › carboxylic acid Boiling Points • Solubility: a solute’s ability to dissolve in a polar solvent (water) – the more polar a substance is, the more soluble it is – solubility decreases as chain length increases – smaller alcohols, aldehydes, ketones & carboxylic acids are typically soluble