Chap 22
advertisement

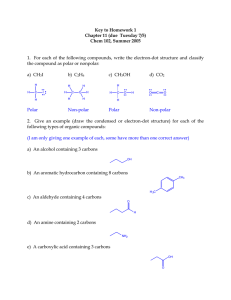
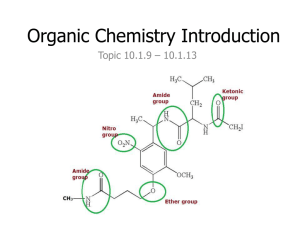
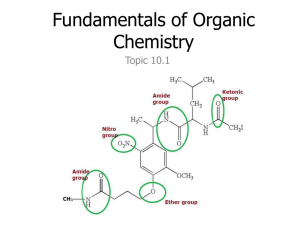
Chapter 22 • Organic Chemistry • Alkanes – all single bonded carbons • Alkenes – at least one double bond • Alkynes – at least one triple bond • Isomers – same number of carbons but different arrangement (structure) • Naming • Based on longest chain of carbons 1 C – Meth 6 C – Hex 2 C – Eth 7C – Hept 3 C – Prop 8 C – Oct 4 C – But 9 C – Non 5 C - Pent 10 C - Dec • Alkanes end with ane • Alkenes end with ene • Alkynes end with yne • Ex. CH3-CH2-CH3 Propane • Ex. On Board • Functional Groups – special attatchements and modifications to the basic carbon chemical • More than one functional group can exist on a single chemical • Alcohols have an OH • Carboxylic acids have an OOH • Ethers have at O trapped between carbons • Esters have a trapped O and a double bonded oxygen see pg 1058 • Aldehydes have a double bonded O at the end of the chain • Keytones have a double bonded O in the middle of the chain • Amines have an NH2 at the end of the chain • Halohydrocarbons have a halogen • Naming • We give the number of the carbon that the functional group is located at, or the first carbon