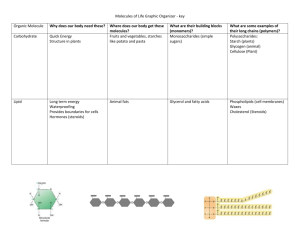
File - Anatolia Biology Class

Unit 2: Chemistry
Today’s Agenda
1. Review flashcards and yesterday’s info.
2. New lessons: Unit 2 Lesson 5 to Lesson 8
3. New flashcards
4. Mid-Unit 2 Review Sheet
5. Organic Compound homework
6. SpongeBob homework
Review
• Remember: Hemoglobin and calcium we talked about are proteins.
Unit 2 Lesson 5: Ions in Living Things
• Living things require ions for many bodily functions.
• Ions play critical role in many of your body’s processes, including the transmission of nerve signals that allows you to clap.
• Ions are often electrical signal of cellular communication.
• The surface of your body carries electrical charges.
• Electrical charges are also distributed inside your body in various ways.
• An ion is an atom with positive or negative electrical charge.
• Ions are present throughout your body and they are distributed in different ways.
• The electrical charges of ions keep your heart beating and facilitate the transfer of messages in your body at high speeds.
• The stability and the way electrical charges are distributed are key components to any living things.
Unit 2 Lesson 5
• Ions play critical roles in living things.
• Many types of ions are extremely important to the survival of living things.
• When you ingest sodium chloride (food with salt), you’re ingesting an inorganic compound- it doesn’t contain carbon.
• Note: Organic compounds HAVE carbon but inorganic compounds DO NOT have carbon.
• Sodium chloride is a compound not formed from carbon-hydrogen bonds; more generally, it’s a compound not produced by a living things. Your ingesting an ionic compound. (a compound made from two atoms bonding through the ionic bond)
Unit 2 Lesson 5
• Example of compounds in play: When table salt dissolves in water or in your mouth and stomach, its atoms separate into ions Na+ and
Cl-
• These ions, each with an electrical charge, now become active in your body in different ways.
Cont.
• Your body uses calcium in several ways.
• Calcium plays an important role in development of bones and teeth.
• Calcium is also critical to function of muscles, clotting of blood after an injury, the transfer of nerve messages through your body, and regularity of your heartbeat.
• It helps regulate substances that cross cell membranes.
• In your body, calcium exists as the ion Ca2+
Cont.
• Carbon dioxide dissolves into bicarbonate ions.
• Two animals are running after each other.
• Both animals are breathing heavily.
• Large amounts of carbon dioxide (CO2) are being generated in the two animals’ bodies, much of which is breathed out as carbon dioxide gas.
• Some CO2 in their blood is converted into bicarbonate ions
(HCO3/2-)
• This conversion must happen to keep acid balance of their body fluids at a level that allows the cells to function properly.
• Bicarbonate ions serve as buffers that keep the acids at levels that cells can tolerate.
• What characteristic of life is this?
Cont.
• Sodium is vital to the balance of water in cells.
• An organism’s cells are filled with fluid and they are also bathed in fluid.
• Balance of water moving into and out of cells is critical to the survival of any living thing.
• Too much water in cells cause them to cease to function.
• Sodium ions play an important role in balancing movement of fluids into and out of cells.
Cont.
• Potassium ions help regulate the heartbeat.
• A hummingbird’s heat beats approximately 1200 times per minute when the bird is feeding.
• Potassium ions (K+) are critical components in that task.
• They play key role in how materials move into and out of cells- including heart muscle cells.
• Potassium ions keep other muscles working properly and act as messengers between muscles and nerves.
• They are also important in keeping blood pressure regulated.
Cont.
• Chloride ions are important to cell balance.
• Do you know what yellow spots mean on plants?
• It means the soil is deficient in chloride ions.
• Chlorine is a negatively charged ion that living things need.
• Chlorine ions (Cl-) have many functions in plants.
• They are vital in opening and closing of stomata- structures on the underside of leaves that allow gas exchange.
• They are also involved in the uptake of nitrogen and in the balance of fluids throughout the plant.
• In animals, chloride ions are most often present in the fluid outside of the cells and in the blood.
• They play similar roles to their positively charged partners, and together they move back and forth, balancing blood volume, blood pressure, cellular fluids, and pH levels.
Cont.
• Hydrogen ions are used in aerobic respiration and to maintain the balance of acids and bases: A monarch butterfly weighs about .5 grams, but this fragile animal can fly from the US to its wintering home in Mexico- about
2000 miles.
• Where does this small thing get the strength to fly that far?
Deep with in the monarch’s cells, large quantities of the energy-donating chemical adenosine triphosphate ATP are produced.
• A complex process takes place within cells to make this energy available.
• Near the end of the process, hydrogen ions (H+) play an important role.
• Without the hydrogen ions, ATP could not be generated in this complex process which is cellular respiration.
Cont.
• Hydrogen ions are also central players in maintaining an acid-base balance in an organism.
• It readily binds to other compounds helping keep the levels of acids and bases regulated.
Cont.
• Several types of ions are critical to living things.
• Many types of chemical compounds are necessary for living things.
• Carbs, proteins, and fats are important and so is oxygen gas and carbon dioxide gas.
• Equally important are certain kinds of ionic compounds and ions they release when dissolved in water.
• The power of these ions comes from the fact that they are electrically charged.
• Their electrical charge allows them to play critical roles in the body.
• Ions like Na+, Ca2+, K+, and Cl- play key roles in the body, including helping regulate what gets into and out of cells, and keeping acids and bases balanced in the body.
Unit 2 Lesson 6
• Many chemical compounds from living things can benefit human health.
• Chemical compounds are used for treating ailments such as: cancer, pain, malaria, heart disease, infection, and high blood pressure.
• Different cultures have used natural compounds for health purposes for thousands of years.
• Humans have been using natural compounds to treat ailments since before recorded history.
Unit 2 Lesson 6
• Scientists isolate and study chemical compounds in living things.
• Scientists determined the effects of thousands of specific molecules that come from plant substances.
• Natural compounds can be beneficial and harmful to humans, and chemists must first alter them to remove harmful substances.
Unit 2 Lesson 6
• Aspirin is made from natural compounds in willow trees.
• Aspirin is a most widely used drug.
• Ancient Greeks and Native Americans used willow tree bark as a pain reliever.
• Aspirin reduces pain, lowers fewer, halts inflammation, reduces risk of heart attack and stroke.
• The chemical compound responsible for those benefits is salicylic acid- (C7H6O3).
• It interrupts a chemical reaction with in cells that produces a kind of molecule found in people experiencing pain.
• This acid prevents the chemical reaction and rids the body of unwanted molecules.
Cont.
• Many natural compounds are used to fight cancer.
• Scientists have developed many cancer fighting drugs from natural compounds.
• One example is Taxolo- C47H5NO14
• It helps stops the spread of cancer.
• Another compound: Ellagic acid in strawberries, raspberries, grapes, and walnuts stops the growth of cancer cells.
Cont.
• Quinine is a medication used to treat malaria.
• It is made from a plant compound.
• Quinine comes from the bark of a cinchona tree.
• French scientists isolated quinine
(C20H24N2O2) in 1820.
Cont.
• Penicillin fights bacterial infections; it comes from fungus.
• In 1928, Scottish scientist Alexander Fleming performed a series of experiments that proved that fungus had the ability to kill kinds of harmful bacteria.
• He discovered penicillin.
Cont.
• Reserpine is a medication for high blood pressure and comes from a plant.
• In the 1930s, scientists studied Indian snakeroot that villages in India used for epilepsy, insomnia, mental disorders.
• Scientists found certain chemicals in plant dramatically reduce blood pressure.
• It was reserpine (C33H10N2O9).
Cont.
• Aloe Vera plants contain compounds that may have health benefits.
• People use aloe vera plant to treat skin conditions.
• Some medicinal compounds come from plants that are poisonous in their natural state.
• A plant called foxglove contains chemicals called digoxin which is a poison and medicine.
• Proper amounts can be broken down in the body into another compound: digitoxin.
• A slight overdose of this compound can be fatal.
Cont.
• Some natural substances can be dangerous if misused.
• Powerful compounds made from ephedra plants have been used successfully for 1000s of years to treat respiratory ailment, but it can also be misused so debate on this drug continues.
Cont.
• Loss is species is loss of potentially beneficial compounds.
• Years of scientific experiments have yielded a variety of medicines we use today, but there are many more to be discovered and studies.
• But as the world’s population increases, species are becoming extinct as their habitats are destroyed.
Review Of Lesson 6
• Humans can benefit from chemical compounds in living things.
• A compound produced by a plant of animal for its own protection can become a potent medicine for humans.
Unit 2 Lesson 7: Water
• Water is essential to living things.
• It has a unique chemical structure.
• Living things depend on water in many ways.
• Living things are composed mostly of water.
• Every cell of living thing is like a water filled factory, providing perfect medium for all life processes to take place.
• It’s at cellular level that most critical reactions occur: form atoms of proteins, transmission of chemical messages, and generation of usable chemical energy.
• Materials in living things are circulated and transported by water based fluids.
• The process of reproduction depends on water.
Cont.
• Water is a polar molecule (electrical/liquid).
• Water molecules are shaped something like miniature boomerangs.
• Hydrogen atoms line up at the angle to the oxygen atom.(Diagram on next slide)
• A water molecule has 2 hydrogen atoms (H) covalently bonded with 1 oxygen atom (O).
• The large oxygen atom with 8 protons pulls more strongly on shared electrons that hydrogen atoms do.
• Electrons are located more near the oxygen atom than the hydrogen atoms.
• This arrangement gives the oxygen atom a slight negative charge and hydrogen atoms slight positive charge.
• This type of molecule, one that has unequal distribution of electrical charges, is a polar molecule.
Water Molecule
Cont.
• Water molecules are joined by hydrogen bonds.
• Why is it called a hydrogen bond?
• When several water molecules bond together, they don’t do so randomly.
• Because water is a polar molecule, the electrical charges align in certain positions.
• Negatively charged oxygen atoms attract the positively charged hydrogen atoms, forming a hydrogen bond.
• The hydrogen bond is weaker than either the ionic bond or covalent bond.
Cont.
• Cohesion and adhesion are the results of hydrogen bonding.
• On molecular level, water molecules stick to each other and many other things.
• The tendency of a water molecule to stick together is cohesion.
• Cohesion is an attraction between same kinds of molecules and substances.
• When molecules of water are surrounded by other water molecules, they’re equally pulled in all directions because of the electrical attraction between hydrogen and oxygen atoms.
• Since water molecules have electrical charge, they’re also attracted to any other substance or material whose molecules have any hint of electrical charge.
• This phenomenon is adhesion.
• You can observe adhesion in action when you see a surface covered with condensation such as a spider web covered with dew.
• The spider web has little electrical charge and partially charged water molecules are attracted to it.
Cont.
• Water surface tension is a result of cohesion.
• A spider watches its prey ready to attack, suspended on the surface of a pond. How is it possible?
• Its possible because of water’s polar nature.
• When a molecule of water is surrounded by other water molecules, its equally pulled in all directions by hydrogen bonds between the molecules.
• But water molecules on the surface have no water molecules above them, so they are attracted to molecules next to them and below them.
• This gives the bonds of top layer of molecules a particular strength.
• This is surface tension. (Surface tension is the result of hydrogen bonding).
• Remember: Adhesion is the attraction between molecules of one substance and the molecules of another substance. Cohesion is the attraction between molecules of the same substance.
Cont.
• Cohesion and adhesion play role in movement of water in plants.
• Within trunks of trees and stems of plants are narrow tube like structures that serve as conduits for carrying water and nutrients from the roots to the rest of the plant.
• Because of water’s adhesive properties, water molecules inside the tubes are attracted to tubes in the inner surface.
• Because of water’s cohesive properties, the rest of water molecules are pulled along with them.
Cont.
• Water is an universal solvent.
• The actions of all living things takes place in the environment that’s basically water, an aqueous environment.
• Water in these environments is a solvent.
• A solvent is a material that causes another to dissolve into it to make a solution.
• The dissolved material is the solute.
• Seawater is a solution. How? Well in the seawater solution, solvent is the water and many other things are solutes that are dissolved in it.
• Because of its polar nature, water can dissolve many materials extremely well.
• Water is nature’s universal solvent.
• Water dissolves more types of solutes than any other substance on earth.
• Easy example: Water and sugar- Water is the solvent since it is the liquid that the solute-sugar, will be dissolved in. Sugar is the solute because it is the material being dissolved in the solvent. The sugar dissolved in the water together makes a solution.
Cont.
• Cellular environment is aqueous.
• Living things need so much water because of the structure of the cell environment.
• Cells are filled with fluid, and fluid is aqueous.
• The energy you need is generated in aqueous environment of your cells.
• Living things have adapted different ways to keep the proper balance of water.
• Cells must maintain certain levels of water at all times. A plant cell stores excess water in a structure called a central vacuole, which releases water as needed.
• All living things must keep a proper balance of water so their cells can maintain aqueous environment.
Review Of Lesson 7
• Remember: Solvent +solute=solution
• Water is a critical component of all living things largely because of its unique physical characteristics and properties.
• Without water, life isn’t possible.
• Living things are made of cells and cells are made of mostly water.
• The chemical structure of water is the polar molecule.
• Keep the right level of water and dissolved materials is necessary for cellular processes to occur properly.
Unit 2 Lesson 8: Biological Compounds
• Chemical indicators help your detect the presence of certain macromolecules in different materials.
• Scientists use various chemical indicators to test for organic compounds.
• Chemical indicators allow scientists to identify compounds in materials.
• Scientists who need to identify the presence of organic compounds may use chemical indicators.
• These various chemicals undergo a color change in the presence of certain macromolecules.
Cont.
• Specific chemicals are used to identify each type of macromolecule.
• The first indicator you can use is iodine.
• In the presence of starch (what macromolecule does starch come from?) iodine solution will turn from yellowish color to dark blue.
• To test for presence of proteins, you can use a combination of sodium hydroxide and copper sulfate.
• A color change will indicate the presence of proteins.
• The third indicator is the chemical Sudan III which is used to test fats. (What macromolecule do fats come from?)
Unit 2 Lesson 9: Simple Carbohydrates
• Simple carbohydrates are made of one or two sugar molecules and are often used as an energy source for living things.
• Carbs are organic compounds made from carbon, hydrogen, and oxygen.
• There are equal numbers of carbon and oxygen atoms, and two times as many hydrogen atoms.
• The building blocks of carbs are single sugar molecules: monosaccharides.
• The most common type of monosaccharide is glucose.
• Single sugars like glucose usually have 5-6 carbon atoms that are formed in a ring structure.
• To draw these sugars, shapes such as pentagons and hexagons are used and at each corner of the shape there is a carbon atom.
Glucose Molecule
Cont.
• Simple carbs are often used as a source of energy.
• Living things use carbs as a source of chemical energy.
• After ingesting them in different forms, living things digest and break down carbs through a series of chemical reactions.
• In all cases, food is broken down into glucose which is a monosaccharide that provides chemical energy for living things.
• Glucose is the main carbohydrate converted into energy by living things.
• Glucose is central to all living things.
• The chemical formula for glucose is C6H12O6
Cont.
• Fructose is also a common monosaccharide found in fruits.
• It has the same chemical formula as glucose but it exists in different structural forms than glucose.
• After being ingested, fructose is converted into glucose so it can enter energy generating reactions like cellular respiration.
Cont.
• Deoxyribose and ribose are also monosaccharides that are found in
DNA and RNA.
• Genes, which transfer traits from parent to offspring, are part of an organism’s DNA.
• Important components of molecules DNA and RNA are sugars
• These important component sugars include deoxyribose and ribose which each have 5 carbons atoms. (Remember, the presence of carbon makes a compound organic, so since carbs are organic and glucose are carbs, glucose/fructose/deoxyribose/ribose are all organic compounds)
• Nucleic acids are molecules that contain and implement genetic control over the cell.
• DNA contains instructions for building proteins; RNA is important to synthesis of those proteins.
• Monosaccharides are essential parts of both RNA and DNA.
Cont.
• Disaccharides are another kind of carbohydrate.
• Sucrose is an example of a disaccharide.
• A disaccharide is a result of two monosaccharides forming a chemical bond. That is why a single sugar is MONO=1 and two sugars bonded is DI=2.
• Sucrose is a table sugar; it is a sweetener.
• Sucrose makes sweet things sweet.
• Sucrose is a glucose and fructose molecule bonded together.
• Carbohydrate made of 2 sugars like sucrose is a disaccharide.
• Rare plants make sucrose like sugar cane and sugar beets- these are very important commercially.
• Sucrose is harvested naturally from sugar cane and sugar beets.
• Note: A monosaccharide is a simple carb while a disaccharide is a complex carb.
Cont.
• Lactose is also a disaccharide found in milk products.
• All mammals produce lactose for their offspring in the form of rich milk.
• Also called milk sugar, lactose is formed when a glucose molecule and galactose molecule form a chemical bond.
• A galactose molecule is another carbohydrate- a monosaccharide that bonds with another monosaccharide to form a disaccharide which in this case is lactose.
Review of Lesson 9
• Simple carbs are often used as a source of energy.
• All carbs contain carbon, hydrogen, and oxygen in the ratio of 1:2:1.
• Simple carbs may bond to one another to make complex carbs.
• The body converts proteins, fats, and carbs into glucose, to produce usable energy for cells.
Unit 2 Lesson 10: Complex
Carbohydrates
• Complex carbohydrates provide energy storage and structure for living things.
• Complex carbs are polysaccharides and are made up from many simple carbohydrate molecules linked together.
• Many foods contain organic molecules which are complex carbs. Polysaccharides are complex carbs which include starch, cellulose, glycogen.
• These are large molecules of hundreds of thousands of glucose molecules held together.
• There molecules store energy and provide strength and support to living things.
Cont.
• Plants store glucose as starch.
• Plants often make more glucose than needed.
• Plants store extra glucose as a molecule starch.
• Starch is a complex carb, a polysaccharide, made of glucose. Starch is stored chemical energy inside a plant.
• Plants store glucose when its abundant.
• When energy is scarce, they break down starch, releasing glucose that become available for the process of cellular respiration.
• When you eat plants where starch is stored, you each this starch.
• Your body breaks it down into individual glucose molecules.
Cont.
• Most starch molecules have a branching structure.
• Different starches show different patterns of branching, in which one chain of glucose molecules branches off of another.
• This type of branching relates to function of starch as a storage molecule.
• In times of energy shortage, enzymes attack the ends of the starch molecule, releasing glucose molecules one at a time for use by cells.
• Branches of glucose chains provide more ends where enzymes can break brown and digest starch quickly releasing more glucose molecules.
• Then they enter cellular respiration, a process which breaks down glucose into chemical energy.
Cont.
• Animals break down starch for energy.
• Your body can break down starch molecules in food to release glucose molecules you rely on for energy.
• Your body produces enzyme molecules.
• Enzymes like amylase break types of chemical bonds that link glucose molecules together in starch molecules.
• Then its free to enter chemical reactions that transform the chemical energy in a glucose molecule into forms of energy your body can use for growth.
Cont.
• Cellulose gives plants strength and support.
• Cellulose is made of glucose molecules. It is a complex carb, a polysaccharide.
• Plants use cellulose as structural molecules.
• It forms the cell wall that gives plant cells shape and support.
• Glucose molecules in cellulose are held together with another type of chemical bond than the glucose molecules in starch.
• This bond is difficult to break down, making cellulose the ideal structural molecule.
Cont.
• Most animals cannot digest cellulose.
• Cellulose and starch is made up of glucose but have different properties.
• Animals mostly can’t use cellulose as energy cause they don’t have enzymes to break the bonds holding the molecule together.
• But its still an important part of your diet.
• Cellulose is a dietary fiber.
• Fruits and veggies are a source of dietary fiber cellulose.
• Its important for your diet because it is not digested.
• It passes through your digestive system and helps your system function effectively.
Cont.
• Some bacteria and fungi can digest cellulose.
• Some species of fungi produce enzymes that break down cellulose.
• These fungi play an important role in recycling dead plants.
• Termites have bacteria in their guys that break down cellulose.
• Bacteria in their stomachs produce cellulose digesting enzymes.
• Without those bacteria, termites would not be able to digest wood and get energy and nutrients from it.
• Animals like cows are also home to may types of cellulose-busting bacteria, which live in their stomachs.
Cont.
• Animals also store glucose in complex carbohydrate called glycogen.
• Animals including humans store glucose in a molecule called glycogen. It’s made of many glucose molecules bonded together.
• Glycogen is stored mainly in muscle cells and liver cells.
• Glycogen is easily broken down during times of energy demand.
• Those glucose molecules can be further broken down, so their stored energy can be converted into usable forms.
• One type of plant produces glycogen.
• The cecropia tree in Costa Rica produces glycogen.
Review Of Lesson 10
• Main Idea: Complex carbs provide energy storage and structure for living organisms.
Unit 2 Lesson 11: Lipids
• Lipids store energy, form cellular membranes, and transmit chemical messages.
• Lipids are organic molecules that don’t mix with water to form solutions. (What is the formula for solutions?)
• Oil is one example of a lipid, a type of organic molecule that generally repels water. (Water, oil, and syrup example)
• Lipids are hydrocarbons, a class of organic molecules made almost entirely of hydrogen and carbon.
Example
Cont.
• Lipids are mainly nonpolar and hydrophobic.
• Lipids consist of long chains of carbon atoms flanked by hydrogen atoms.
• Lipids are hydrophobic: water avoiding.
• They are not electrically charged; neither positive nor negative. This explains why they do not attract to water molecules that are both negatively and positively charged. Remember water molecules are polar.
• Lipids are nonpolar; no attracted to polar molecules with water.
• But this is the tail of the molecule. Only the tail of the lipid is nonpolar.
• The head is attracted to polar water molecules.
Cont.
• Fats and oils are energy storage lipids.
• Fat is lighter in weight than carbs and the given volume of fat contains twice as much chemical energy as the same volume of carbs.
• All stored fat is converted to glucose.
• Fat is excellent for storing chemical energy.
• Fats and oils store energy in carbon hydrogen bonds.
• Fats and oils are types of lipids called triglycerides that comprise of two types of chemical bonding blocks: a small glycerol molecule and 3 small fatty acid molecules.
• A fatty acid is a long chain of carbon and hydrogen atoms that has clusters of carbon, oxygen, and hydrogen at one end.
• Fats are triglycerides that are solid at room temperature.
Cont.
• Fats and oils contain more energy-rich carbon/hydrogen bonds than carbs.
• The breakdown of fats and oils produces more energy in the form of ATP than the break down of glucose.
• The first thing that happens is that the body converts these fats to glucose.
Cont.
• Saturated fats have single bonds between carbon atoms.
• Saturated fats are found in animals and animal food you eat.
• The carbon atom has space to form 4 chemical bonds with other atoms.
• A saturated fat is made up of fatty acid chains with single bonds between each carbon atom.
• In the chain, two bond spaces are taken up by other kinds of atoms.
• The two other bond spaces are filled with hydrogen atoms, and the fat is said to be saturated.
• That arrangement makes long and straight fatty acid chains, which can easily pack together.
Cont.
• Unsaturated fats have double bonds between carbon atoms.
• Unsaturated fats are found in plant products.
• They have double bond between some carbon atoms in the fatty acid chain.
• That double bond takes up two available spaces, leaving space for only one atom on a double bonded carbon atom.
• Because hydrogen atoms don’t fill all available spaces, its an unsaturated fat.
• Double bonds produce bends in the fatty acid chain.
• They keep unsaturated fats from stacking as smoothly as saturated fats.
• Unsaturated fats like olive oil, corn oil, are liquid at room temperature.
Fats
Unsaturated
Saturated
June 16th
Cont.
• Phospholipids form cellular membranes.
• The cell is key to maintaining homeostasis.
• The outer membrane of a cell comprises a dual layer of lips called phospholipids.
• The structure of this layer helps the cell maintain constant internal environment.
Cont.
• The cell membrane is a phospholipid bilayer.
• A phospholipid is equal to a fat: it contains fatty acid chains and glycerol.
• But phospholipid bilayer contains only 2 fatty acids while fat contains 3 fatty acids.
• The phospholipid bilayer also has a chemical structure called a phosphate group.
• This gives one end of the phospholipid a negative electrical charge: this end of the chain is hydrophilic because it attracts water molecules.
• In the watery environment, phospholipids arrange themselves so the fatty acid tails which are hydrophobic, point inward toward each other, while phosphate groups (hydrophilic heads) point out, interfacing with water.
• This layer creates two layers of phospholipid- a bilayer.
• The phospholipid bilayer makes up the outer membrane of all the cells in your body.
• The phospholipid bilayer can contain a separate internal aqueous environment relative to the outside.
Cont.
• Steroids are lipids that include ring structures.
• Cholesterol is a steroid which is important to many cellular structures.
• Your body uses cholesterol to make steroids like estrogen.
• Steroid hormones are chemical messengers.
• Your body produces a steroid hormone called cortisol in response to stress.
• One effect of it is to make you sick when you are stressed.
• Cortisol is a chemical messenger that carries info from one part to another part of the body.
Cont.
• Wax repels water because it has a waterproof coating.
• Wax is a type of lipid made of extremely long chains of fatty acids linked to an alcohol molecule.
• Fatty acids in waxes are saturated so they pack well together.
• Fatty acid chains are nonpolar, so they don’t attract water, which makes wax hydrophobic or water-repellent.
Main Idea
• Lipids store energy, form cellular membranes, and transmit chemical messages.
Unit 2 Lesson 12: Amino Acids and Proteins
• Amino acids bond together to form different shaped proteins.
• A protein’s shape affects its function.
• Amino acids are important chemical building blocks of all proteins, which are large molecules with many functions.
• Amino acids share a chemical backbone of carbon, hydrogen, nitrogen, and oxygen.
• Amino acids have a central carbon atom with 4 components attached.
(Remember, the carbon atom has 4 spaces available to bond)
• 3 of these components are identical in all amino acids: 1 hydrogen atom, an amino group that holds a nitrogen amino and 2 hydrogen atoms, and a carboxyl group of a carbon atom bonded with 2 oxygen atoms, one of which also bonds with a hydrogen atom.
• The 4 th unit that is connected to an amino acid is an R-group: a side chain.
• This R group is a variant, in another words, it varies in every amino acid.
• Its chemical and physical properties give amino acids unique characteristics and functions.
• If not for the R-group, each amino acid would be the same.
3 of these components are identical in all amino acids: 1 hydrogen atom, an amino group that holds a nitrogen amino and 2 hydrogen atoms, and a carboxyl group of a carbon atom bonded with 2 oxygen atoms, one of which also bonds with a hydrogen atom.
The 4 th unit that is connected to an amino acid is an R-group: a side chain.
This R group is a variant, in another words, it varies in every amino acid.
Amino
Group
H
Cont.
• About 20 amino acids that exist in nature make up all proteins in living organisms.
• Some R groups are non polar and hydrophobic, while some are polar and hydrophilic.
• Some have negative and positive charge (what is this called?); these are attracted to water.
• Proteins play roles in structure and support, metabolism (Define?), homeostasis (define?), and defense from disease and etc.
Cont.
• Amino acids combine to form peptides.
• Amino acids bond forming a structure called a peptide: consists of 2 or more amino acids.
• This bond forms when an enzyme that speeds up chemical reactions, causes a dehydration reaction.
• In this, the –OH end of carboxyl group joins with a hydrogen atom from the amino group.
• This reaction make molecules of water and peptide bond between 2 amino acids.
• This reaction can form many times to form a polypeptide, which is long chains of amino acids.
Cont.
• A protein is made up of 1 or more polypeptide chains.
• The chains twist and fold to give protein a complex shape and structure.
• Those physical characteristics affect how proteins function in living things.
• Proteins perform many functions.
• Enzymes are just one type of protein. (What is the function of an enzyme?)
• Proteins also provide support, structure and storage, help maintain homeostasis, defend from disease, and help carry out important molecules through the body.
Cont.
• Proteins provide structure and support.
• For example, keratin is a protein that makes up your hair and nails.
It also forms birds’ beaks and feathers and a reptile’s scales, etc.
• Collagen is another protein that makes up bones, teeth, cartilage, and tendons.
• Proteins also provide storage and transport materials through your body.
• The protein hemoglobin (Recall: What organic compound does hemoglobin include?) in your blood picks up oxygen in your lungs and delivers it through body.
• Each hemoglobin molecule contains iron which binds to oxygen.
• Hemoglobin is a transport protein.
• Your body uses iron to make hemoglobin, but your body can also store iron for future use in a storage protein called ferritin; it stores iron for times in need of iron.
Cont.
• Proteins help organisms respond to the environment.
• Proteins actin and myosin work together to cause muscles to contract and move.
• Receptor proteins found in cell membranes recognize and bind to specific molecules like hormones, which are chemical signals released by the nervous system.
• Other proteins help transport materials across the lipid bilayer of the cell membrane.
Cont.
• Proteins defend the body from disease too.
• When you catch a cold, your body learns to recognize that virus that caused you to be ill.
• You won’t catch it again because your body recognizes it an can mount attack against it.
• This is immunity your body has built comes from proteins called antibodies.
• Antibodies are defense proteins that the body produces in response to invading pathogens, like bacteria and viruses.
Review of Lesson 12
• Proteins are made of amino acids.
• Amino acids are made of polypeptide chains.
• Proteins are essential to your body in the sense that they provide support, structure, and storage, help maintain homeostasis, defend your body from diseases, and help carry out many important molecules throughout your body.
Unit 2 Lesson 13: Levels of Protein
Structure
• Proteins have 4 levels of structure: primary, secondary, tertiary, and quaternary.
• A protein is a large molecule made of 2 or more peptide chains.
• Each polypeptide is made up chains of amino acids which are molecules or nitrogen, oxygen, carbon, and hydrogen.
• Proteins are defined by 4 structural levels, starting with the order of amino acids that make up the polypeptide chain, continuing up to interactions of chains themselves.
Cont.
• Primary structure is its sequence of amino acids.
• Red blood cells are shaped like squashed sphere, indented in the center.
• People with sickle cell anemia have altered cell shape that interferes with the cell’s ability to travel through the capillaries.
• A change in just one amino acid in one polypeptide chain in the hemoglobin molecule causes this.
• Scientists would say that a change in the primary structure of hemoglobin causes this.
• The primary structure is the order of amino acids that makes up a polypeptide and protein.
• The primary structure plays an important role in protein function.
Primary Structure of Protein
Cont.
• Changes in proteins affects protein function.
• Normal hemoglobin protein has a molecule of glutamic acid.
• The sickle-cell form has valine in its place.
• The proteins molecules contain units called R-groups.
• Glutamic acid is a charged amino acid which means it is hydrophilic.
• Valine is nonpolar meaning it is hydrophobic.
• Both of these acids are a part of the R group in an amino acid.
• The hydrophobic valine of one chain is attracted to another one within the red blood cell.
• This attraction causes the protein to twist in an unnaturral way, causing the whole cell to sickle.
Cont.
• A protein’s secondary structure is formed by hydrogen bonds between amino acids in a polypeptide chain.
• What are hydrogen bonds?
• Some regions of polypeptide chains in the keratin protein take on a twisting shape called an alpha helix.
• That is the protein’s secondary structure.
• Most proteins have 2 types of secondary structures.
• Polypeptide chains take up the shapes of alpha helix or beta pleated sheet.
Secondary Structures of Proteins
Alpha Helix
Beta Pleated Sheet
Cont.
• Proteins tertiary structure takes on bends and folds.
• Protein keratin contain amino acid cysteine which is unusual because it contains sulfur in its R groups.
• Sulfur atoms bond with one another to form disulfide bridges that give extra strength to keratin in the hair.
• This bond gives rise to tertiary structure.
• Polypeptide chains of many proteins bend and fold in certain places, contributing to the overall protein shape.
• Tertiary structure is the bonding of beta pleated sheets and alpha helices as a result of an attraction between them.
Tertiary Structure of Protein
Cont.
• Protein’s quaternary structure is the way that’s polypeptide chains bind and interact.
• Protein is made of two or more polypeptide chains.
• Those chains are called subunits, which interact with one another that brings the highest level of protein structures.
Quaternary Structure of a Protein
Review of Lesson 13
• A protein’s shape influences its activity
• Of millions and millions of proteins that function in living things, each has to have particular shape to perform its function.
• The 4 structures of a protein are:
1. Primary structure: the order of amino acids that makes up a protein or polypeptide.
2. Secondary structure: the coiling or folding of a polypeptide in which amino acids are near one another in the chain bond.
3. Tertiary structure: the folding of a polypeptide with secondary structure in which amino acids are far from one another in the chain bond.
4. Quaternary structure: the association of two or more polypeptide chains in a spatial relationship to make up a protein.
Review Diagram
Unit 2 Lesson 14: Proteins as Enzymes
• Enzymes are proteins that speed up chemical reactions that make life possible.
• An enzyme is a catalyst: a compound that changes the speed of a chemical reaction without being changed by the reaction.
• Enzymes are necessary for maintaining most of the characteristics of life.
• More than 1,500 enzymes identified in human body processes are necessary for life.
• Scientists use the suffix “-ase” to indicate that a chemical is an enzyme.
• They are also grouped based on common activities they share.
• For example, lactase breaks lactose found in dairy products to glucose for the body to digest.
Cont.
• Enzymes themselves are not permanently changed in the reaction.
• Lactase is an enzyme that interacts with lactose.
• This interaction breaks bonds holding the two monosaccharides (define this?) galactose and glucose together, that make up the lactose molecule.
• The structure and function of lactase enzyme remains unchanged during the reacion.
• Once the bond is broken, the lactase enzyme is free to brake more bonds.
• Important concept: enzymes help speed up chemical reactions, but they don’t change themselves as a result of the reaction.
Cont.
• Enzymes recognize and interact with specific molecules.
• Lactase enzymes break apart only the lactose molecule and no other molecule like a protein or fat.
• Another important concept: Enzymes recognize and bind to specific molecules: enzyme specifity.
• This explainss why there are so many enzymes.
• No enzyme is structured to interact with more than 1 type of molecule.
• Each molecule has its own unique and specific enzyme.
Cont.
• An enzyme’s structure is responsible for its binding properties.
• The overall shape, structure, and chemistry of an enzyme is what accounts for its specificity. In other words, its properties determine with molecule it is made for.
• The molecule that the enzyme reacts with is the substrate.
• The region of the enzyme that recognizes the substrate is the active site which appears as an indentation of the enzyme, into which the substrate fits.
• Substrate molecules bind to an enzyme’s active site to produce an enzyme-substrate complex.
• Then the enzymes grasps on the substrate more tightly.
Enzyme Substrate Complex
Cont.
• The specific shape and structure of an enzyme’s active site determine molecules with which it can interact.
• The enzyme catalyses the reaction, like braking apart the substrate molecule.
• The end result of enzyme-catalyzed reaction is the product.
• When a molecule is broken, the products leave the enzyme to interact with another molecule.
• In a given enzyme, the active site has a specific shape and structure into which only certain molecules can fit.
Product
Cont.
• Enzymes bring molecules together.
• Enzymes hold molecules in specific orientations, allowing 2 molecules to form a chemical bond that wouldn’t normally form.
• Enzymes help ensure that the correct regions of molecules interact with each other.
Cont.
Cont.
• Enzymes lower a reaction’s activation energy.
• The amount of energy required for the reaction to take place is the activation energy.
• Enzymes speed up chemical reactions by lowering activation energy.
• For ex, bringing molecules together in just the right way helps lower the amount of energy that would otherwise have been required to bond to form if those molecules were moving freely in space.
Cont.
• Enzyme-assisted reactions reach a max rate.
• That point occurs when all enzymes are bonded to substrate molecules.
• At that point, enzymes are said to be saturated: when this happens, the reaction does not proceed any faster: its at its max rate.
• Some enzymes need other molecules to work properly.
• In your body, vitamins help produce small molecules called coenzymes, which some enzymes require to function properly.
• Coenzymes bond with one enzyme, then move on and interact with more.
• Certain enzymes also require cofactors.
• Coenzymes and cofactors help enzymes by performing their function.
Coenzyme
Cont.
• An enzyme’s environment influences its activity.
• Each enzyme has specific conditions ideal for its activity; changing those conditions may affect the enzyme’s activity.
• The temperature and PH level affects enzymes.
• Alterations in the shape of the enzyme may prevent it from binding with a substrate.
June 17th
Unit 2 Lesson 15: Nucleic Acids
• Nucleic acids are large organic molecules that store and transmit genetic info and play a central role in building proteins.
• Nucleic acids are macromolecules that store and implement genetic info.
• All living things contain nucleic acids, which provide instructions for an organism’s growth and development.
• DNA contains an organism’s genetic info, which is passed from generation to generation, while RNA uses instructions provided in DNA to build proteins.
Cont.
• DNA is shaped like a twisted ladder: it is a double helix consisting of two strands of nucleotides linked together.
• DNA molecule consists of 2 strands of individual nucleotides linked together.
• See diagram on next page.
DNA Double Helix Molecule
Cont.
Nucleotide
Cont.
• Nucleic acids are made up of repeating units of 5 carbon sugars, phosphate groups, and nitrogenous bases.
• Nucleic acids are made of smaller units called nucleotides: molecules that share a general chemical structure.
• A nucleotide is a small molecule made of 5 carbon sugars, a phosphate group, and a nitrogenous base.
• A phosphate group is made up of atoms of phosphorous and oxygen.
• A nitrogenous base is a structure containing nitrogen.
• Both DNA and RNA are formed when the sugar of one nucleotide bonds with the phosphate group of another nucleotide.
• Multiple nucleotides bonded together form a sugar phosphate back bone.
Cont.
• Nucleic acids contain several types of nitrogenous bases.
• The sugar and phosphate group in the molecule DNA are identical from one nucleotide to the next.: the sugar is always deoxyribose and the phosphate group is always phosphorus and oxygen.
• This is the same for RNA, except that the sugar in every nucleotide is ribose instead of deoxyribose.
• However, in each nucleic acid, nitrogenous bases may differ from one nucleotide to another.
• DNA contains 4 nitrogen bases adenine, thymine, cytosine, and guanine.
• They are classified into groups.
• Adenine and guanine are purines.
• Cytosine and thymine are pyrimidines.
• Purines are bases with 2 rings of carbon and nitrogen.
• Pyrimidines are bases with just one ring of carbon and nitrogen.
Cont.
• DNA is a double stranded molecule in the shape of a twisted ladder.
• DNA contains genetic blueprints that your body needs for growth and development.
• DNA in your cells divides when cells in your body divide.
• This is replication: producing identical copies of DNA for new cells.
Cont.
• Inside DNA’s double helix are nitrogenous bases.
• These bases line up with each other in a specific way.
• Adenine and thymine pair up.
• Cytosine and guanine pair up.
• This pattern of matching is called “complementary base pairing.”
• Hydrogen bonds form between the nitrogenous bases in a DNA molecule.
• Remember those nitrogenous bases are guanine, cytosine, adenine, and thymine.
• Inside the double helix, a double ring structure always pairs with a single ring structure, stabilizing the helical structure.
• Hydrogen bonds form between the nitrogenous bases: 2 bonds form between adenine and thymine while 3 bonds form between cytosine and guanine.
• These bonds give extra support to the molecule.
Cont.
• The sequence of nitrogenous bases in one strand of DNA determine the sequence in the other strand.
• The knowing the identity of one nitrogenous base in a molecule of DNA, you can predict the identity of its pair in the opposing strand.
• For example, if one strand of DNA is in the following order:
Guanine, thymine, thymine, guanine, cytosine, adenine, adenine, and cytosine, the other strand of DNA would be cytosine, adenine, adenine, cytosine, guanine, thymine, thymine, and cytosine.
• Remember this is this way because guanine bonds with cytosine and thymine bonds with adenine.
• The sequence of bases in a DNA molecule contains information that the organism needs to build proteins and carry out important life processes.
Cont.
• RNA is a single stranded nucleic acid.
• The sugar phosphate backbone of RNA is made of the sugar ribose instead of deoxyribose. That is how you can tell the difference between the two nucleic acids. Deo..= DNA> Ri..=RNA
• RNA uses a nitrogenous base called uracil in place of thymine which is complementary to adenine.
• RNA exists as a single stranded molecule inside of a cell.
• RNA helps cells read the instructions in DNA and use them to make proteins.
• For example, cells contain different types of RNA molecule.
• RNA translates information in DNA into amino acids, which are the building blocks of proteins.
• Cells have 3 types of RNA: messenger, ribosomal, and transfer RNA.
• Each type of RNA is produced during transcription process.
• RNA plays the role of the middleman between the information in a strand of DNA and the protein produced from that information.
Unit 2 Lesson 16: ATP
• ATP is a complex organic molecule that provides energy for all life processes such as growth, development, and response to the environment.
• ATP is a molecule that stores and provides energy for all life’s processes.
• You learned earlier about the 7 characteristics of life.
What are the 7 characs of life?
• Each of these characteristics require energy.
• The chemical energy that powers these processes in living things is the molecule adenosine triphosphate or in other words, ATP.
• ATP is produced during the cellular pathways that break down the molecule glucose.
• Cells produce and use ATP in many different ways.
Cont.
• ATP is made from other chemical components.
• Molecule ATP consists of the nitrogenous base adenine, the sugar ribose, and 3 additional phosphate groups, or units made of a phosphorus atom surrounded by 4 oxygen atoms.
• The tail of phosphate contains 3 phosphate groups: hence the name tri phosphate.
• The unit consisting of adenine and ribose is called adenosine.
Adenine + ribose = adenosine.
Cont.
• The energy of ATP is stored in the chemical bonds in its phosphate tail.
• The tri phosphate tail of ATP molecule is its energy storage house to release energy.
• ATP is broken down from the tip of its tail by the removal of 1 phosphate group at a time.
• This reaction is called hydrolysis.
• Hydrolysis of ATP produces a free phosphate group and a new molecule called adenosine diphosphate ADP.
• That reactions releases energy.
• Its energy drives many chemical reactions in a cell.
• Cells can also make ATP from ADP by using energy to reconnect a phosphate group.
• In this way, ATP is regenerated for use again by a cell.
• The process of connecting that phosphate group is complex.
Cont.
• Cells use and produce ATP.
• The sun shines and a plant makes glucose. What is this process called?
• The energy in the bonds of glucose is transferred to the bonds in an ATP molecule.
• ATP is produced as an end result of breaking down a glucose molecule, which occurs through several different processes. (It occurs through many processes, but which is the main process we talked about that generates
ATP?)
• There is no organisms on earth to make ATP directly. Even plants undergo the process to break glucose into ATP called cellular respiration.
• ATP must be made from energy that is in the bonds of glucose molecules.
• Even the first step in breaking down glucose requires ATP.
• The break down of glucose produces more energy in the form of ATP that it requires.
• All that ATP is used to drive the activities of a cell and must be constantly produced.
Cont.
• Many cellular processes require ATP.
• Cells use ATP to help move molecules, ions, and other substances across their cellular membranes. (What is a cellular membrane? What kind of macromolecule is it made of? What is the specific name of the structure? What is the cellular membrane of a plant cell called and what carbohydrate is it made of?)
• Some substances can flow across a cell’s phospholipid bilayer but energy may be required to bring substances into a cell or out of a cell.
• This is active transport.
• Cells use ATP for 1000s of chemical reactions.
• The breakdown in ATP production will kill a cell.
Cont.
• ATP powers muscles in the body.
• It provides the energy for your muscles to contract.
• It’s impossible for muscle cells to store enough
ATP to power motion that lasts more than a few seconds so ATP is constantly produced in muscle cells as they perform work.
• The processes of cellular respiration and glycolysis(another process for breaking down glucose) provide ATP for your muscles.
Unit 2 REVIEW
• We are done with Unit 2: The Chemistry of
Life.
• Now we will review.
• The Unit 2 Test is TOMORROW!!!
Review Plan
• Review all flashcards for this unit.
• Review the Mid Unit 2 worksheet
• Review the Unit 2 worksheet
• Review all the Mastery Maps
• Review Sheets from Website
• Pages 18-45 in the Biology Textbook