Water the Magic Molecule of Life
advertisement
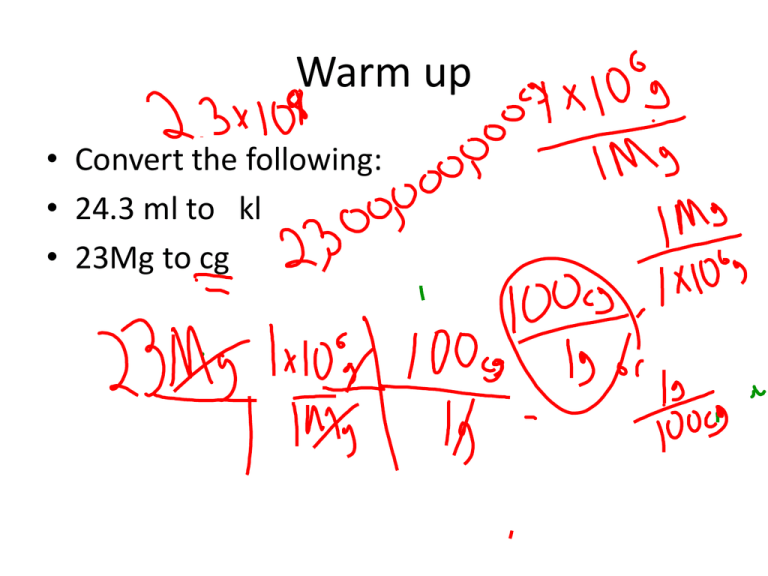
Warm up • Convert the following: • 24.3 ml to kl • 23Mg to cg Water the Magic Molecule of Life The Polarity of Water Molecules • The Unequal sharing of Electrons in a covalent molecule results in a polar molecule Cohesion • This is the attraction of molecules of water to each other due to hydrogen bonding – In plants it helps them pull the water molecules up against gravity Adhesion and Surface Tension Water Has a High Specific Heat • Specific heat- The amount of heat it takes to change the temperature of 1 g of a substance 1°C – Calorie- the amount of heat necessary to raise 1g of water 1°C – Hydrogen bonding dampens the effect of heating thus causing the high specific heat Evaporative Cooling Ice vs Water Ice is less dense thus it is floating an insulating the water below Solvent of Life • Aqueous solutions have water as the solvent – Hydration shell is the hydrogen side surrounding anions and oxygen side surrounding cations in solution – Non-ionic compounds can also dissolve if they have polar regions or ionic bonds on their surface Acidic and Basic Solutions











