March 1 st - Northeast High School

March 1
st
Topics:
1.
Properties of Water
2.
Organic Molecules
3.
Enzymes
Concept
Polarity
Universal Solvent
Density of Water
Temperatures Effects
Water Properties
pH and Scientific Notation
Details
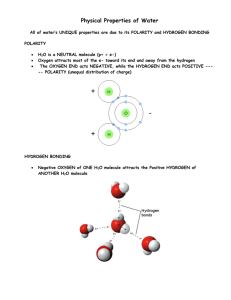
o Polarity means _____________ o Oxygens charge is _____ o Hydrogens charge is _____.
o Water to has _____ charged regions o Water molecules stick together by forming___________ bonds.
o Substance being dissolved _________ o Substance that causes dissolving is ___________ o Water is polar, therefore it dissolves all ________ substances o Blood carries nutrients and minerals needed because it contains _____________ o 100 o C- water is _____ dense (gas) o 20 o C- water is _______ dense than 100 o C (liquid) o 4 o C- water is the _______ dense (liquid) o 0 o C- water is solid and is ____ dense than liquid water o As a solid, water molecules form a crystal hydrogen bonds. Water molecules in ice move
______causing the ice to be less dense and float. o Surface Ice helps organisms to survive through the winter because lake do not freeze _________ o At 100 o C water molecules are moving ________ and are far apart o Liquid water molecules ______down as temperature decreases. o The closer water molecules are the more
__________they are o pH scale 0-14 o Acids are between: _________ o Neutral: 7 o Bases are between : _________ o All chemical reactions have an _____________pH o All living things have an ____________pH o Every whole number on the pH scale represents a
______fold increase. o 100,000 = _______________ o A pH of 11 is 1.0 x 10 5 times greater than a pH of
6.
ORGANIC MOLECULES REVIEW
Directions: The following statements are associated with the four organic molecules.
Use the letters to indentify the organic molecule the statement is referencing. Some statements may be associated with more than one molecule.
C- carbohydrate; L – lipids; P – proteins; N – nucleic acids
1. _________ building block is amino acids
2. _________ main source of energy for cells
3. _________ cellulose makes up cell walls
4. _________ speeds up chemical reactions
5. _________ building blocks are glycerol and fatty acids
6. _________ double helix DNA
7. _________ primary function structural molecule of muscle cells
8. _________ function to store energy and insulate
9. _________ contains carbon, hydrogen, oxygen, and nitrogen
10. _________ phospholipid
11. _________ starch for stored energy
12. _________ molecule in cell membrane structure
13. _________ glucose as quick energy
14. _________ contains carbon, hydrogen, oxygen, nitrogen, and phosphorous
15. _________ enzymes are a important type
16. _________ stores genetic information
17. _________ building blocks are monosaccharides
18. _________ RNA, single stranded form of…….
19. _________ building blocks are nucleotides
20. _________ Type of molecule that contains only elements carbon, hydrogen, and oxygen
All About Enzymes: Factors Affecting Enzyme Activity and other Stuff
Factors Graph Write a statement explaining how the
1 factor affects enzyme activity? pH
2
3
4
Substrate
Concentration
Enzyme
Concentration
Temperature