functional group project
advertisement
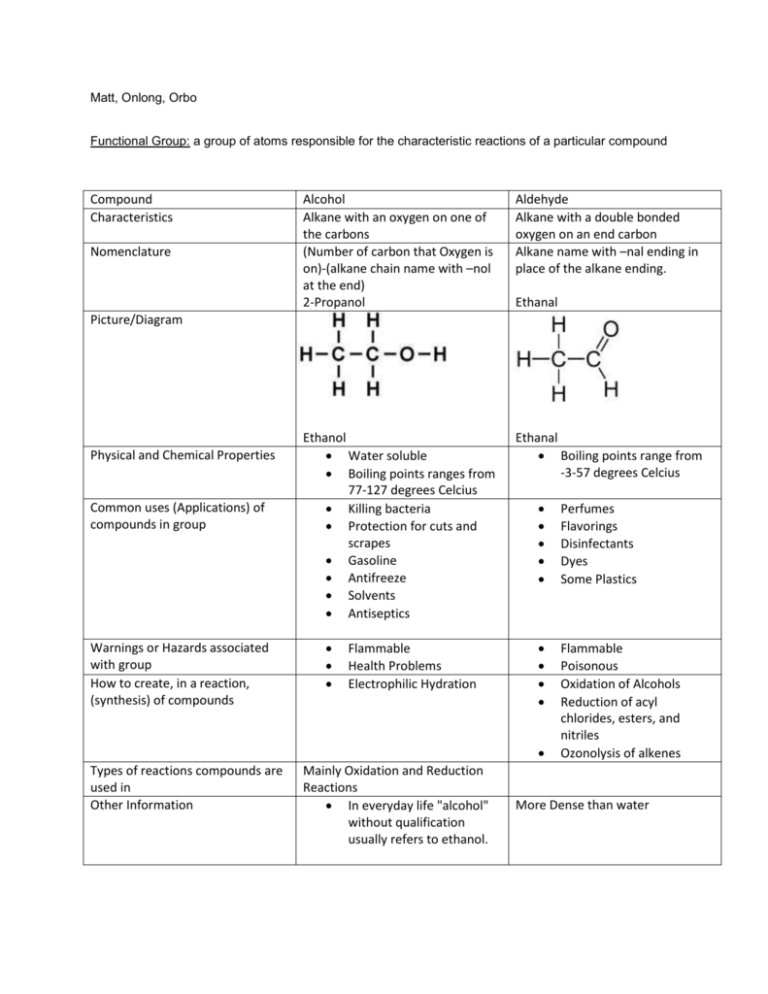
Matt, Onlong, Orbo Functional Group: a group of atoms responsible for the characteristic reactions of a particular compound Compound Characteristics Nomenclature Alcohol Alkane with an oxygen on one of the carbons (Number of carbon that Oxygen is on)-(alkane chain name with –nol at the end) 2-Propanol Aldehyde Alkane with a double bonded oxygen on an end carbon Alkane name with –nal ending in place of the alkane ending. Ethanol Water soluble Boiling points ranges from 77-127 degrees Celcius Killing bacteria Protection for cuts and scrapes Gasoline Antifreeze Solvents Antiseptics Ethanal Boiling points range from -3-57 degrees Celcius Ethanal Picture/Diagram Physical and Chemical Properties Common uses (Applications) of compounds in group Warnings or Hazards associated with group How to create, in a reaction, (synthesis) of compounds Flammable Health Problems Electrophilic Hydration Perfumes Flavorings Disinfectants Dyes Some Plastics Flammable Poisonous Oxidation of Alcohols Reduction of acyl chlorides, esters, and nitriles Ozonolysis of alkenes Types of reactions compounds are used in Other Information Mainly Oxidation and Reduction Reactions In everyday life "alcohol" without qualification usually refers to ethanol. More Dense than water http://licn.typepad.com/my_weblog/2010/12/dielectrophoresis-john-dunn-consultant-ambertec-pepc.html http://chemistry.about.com/od/factsstructures/ig/Chemical-Structures---A/Acetaldehyde-orEthanal.htm http://cameochemicals.noaa.gov/chemical/2330 https://sites.google.com/site/chemistryolp/properties-of-alcohols http://www.britannica.com/EBchecked/topic/13527/aldehyde http://www.ask.com/question/uses-of-aldehydes http://crab.rutgers.edu/~alroche/Ch11.pdf http://en.wikibooks.org/wiki/Organic_Chemistry/Alcohols http://www.cliffsnotes.com/sciences/chemistry/organic-chemistry-ii/aldehydes-and-ketones/synthesisof-aldehydes