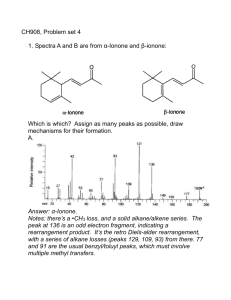
Organic Chemistry
advertisement

Alkanes All end in -ane General formula CnH2n+2 Identify by the C-C bone (single) Saturated Hydrocarbons Alkenes All end in –ene General Formula CnH2n See C=C double bond Alkynes All end with –yne General Formula of CnH2n-2 Identify the triple bond Cyclic Hydrocarbons Also called Aromatic Hydrocarbons Also called Benzene series General formula is CnH2n-6 Benzene C6H6 Cyclic Hydrocarbons Toluene C7H8 Also called Methyl Benzene Steps 1. Identify the longest C-C continuous chain Steps 2. Look for any C=C or C=C Bonds and identify by the lowest Carbon number location Step 3. Look for any Alkyl side chains: (Alkyl groups are Alkanes less 1 Hydrogen) Alkane Alkyl Have the same molecular formula Different structural formula R = an atom or group of atoms in a Hydrocarbon chain X = a Halogen (Group 17) R-X Naming Rule Indicate position of halogen on longest hydrocarbon chain. R-OH Naming Rule Indicate position of -OH group on carbon chain. Change “e” of alkane name to “ol.” R–O–R Naming Rule Name each R group and tack-on “ether.” Naming Rules Aldehydes: Change “e” of alkane name to “al” Ketones: Indicate position of carbonyl Change “e” of alkane to “one” Naming Rules Change alkane “e” to “oic acid” O R – C – O – R’ Naming Rules 1. Name R’ alcohol group 2. Change R alkane “e” to “oate” ***HHH______ Made from combining an Acid and an Alcohol R’ R – N – R’’ Naming Rules Label position of N. Change “e” of alkane name to “amine.” O R’ R–C–N–H Naming Rule Change “e” of R alkane name to “amide” Adding a halogen to an unsaturated hydrocarbon (alkene / alkyne) Yields 1 product! + Br2 Adding a halogen to a saturated hydrocarbon (alkane) Yields 2 products, one an acid! + Br2 Br + HBr A reaction that always yields Ethanol and CO2! Recall: anaerobic Respiration?? A burn reaction (always needs O2) and always yields CO2 and H2O Making of Soap, always yields an Alcohol (Glycerol) and Soap! Look for the 3 NaOH bases!!!! Making Esters Acid + Alcohol yields an Ester and Water + C C ethanol + H2O Making Esters Acid + Alcohol yields an Ester and Water Note the Esters 1. fragrances, sweet smells (bananas) Naming revisit 1. Start with the Alcohol Side 2. Finish with the Acid side The making of polymers 1. Natural – proteins, polysaccharides 2. Artificial – plastics, nylons, rayon n Addition Polymerization Addition Polymerization Condensation Polymerization Always yields Water!