Chemical Bonding
advertisement

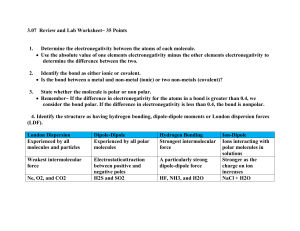
Periodic Table Groups 1. Color-code the following groups on your periodic table: -alkali group -alkaline group - transitional group -halogen group -noble gases -inner transitional group 2. Add the staircase (separates metals/non-metals.) 3. Place an asterisk by each metalloid. 4. Non-metals groups: Which group is most reactive and which is most stable? Explain how you know this. 5. Metal groups: Which group is most reactive and which is most stable? Explain how you know this. 6. Give an example of two elements that will form an ionic bond to reach stability. 7. Give an example of two elements that will form a covalent bond to reach stability. 8. What does each period on the periodic table symbolize for an atom? 9. What period and group is Bromine in? 10. a. What is electronegativity? b. What is the trend in electronegativity across a period and down a group on the periodic table. Chem II-Period: 9.11.14 Infinite Campus: • Scientific Skills and Matter Exam (48pts.) Objectives: • Periodic Table Organization and Trends • Chemical classification and nomenclature Homework: • Chemical Nomenclature Packet(ionic/covalent) Periodic Table Groups 1. Color-code the following groups on your periodic table: -alkali group -alkaline group - transitional group -halogen group -noble gases -inner transitional group 2. Add the staircase (separates metals/non-metals.) 3. Place an asterisk by each metalloid. 4. Non-metals groups: Which group is most reactive and which is most stable? Explain how you know this. 5. Metal groups: Which group is most reactive and which is most stable? Explain how you know this. 6. Give an example of two elements that will form an ionic bond to reach stability. 7. Give an example of two elements that will form a covalent bond to reach stability. 8. What does each period on the periodic table symbolize for an atom? 9. What period and group is Bromine in? 10. a. What is electronegativity? b. What is the trend in electronegativity across a period and down a group on the periodic table. Periodic Table: Organization and Bonding Bell Ringer: Periodic Table Check-up 1. Give an example of an element from each group below: alkali, halogen, transitional, noble gas, and alkaline-earth. 2. Which element from qts. 1 is a metal and is considered to be the most reactive. Explain your answer. 3. Which element from qts. one is a non-metal and considered the most reactive? Explain your answer. 4. Which element from qts. one is the most stable. Explain your answer. 5. Give an example of two elements that would form: a. a covalent bond b. an ionic bond 6. What is electronegativity and how is it related to chemical bonding? Bell Ringer: Periodic Table Check-up 1. Give an example of an element from each group below: alkali, halogen, transitional, noble gas, and alkaline-earth. 2. Which element from qts. 1 is a metal and is considered to be the most reactive. Explain your answer. 3. Which element from qts. one is a non-metal and considered the most reactive? Explain your answer. 4. Which element from qts. one is the most stable. Explain your answer. 5. Give an example of two elements that would form: a. a covalent bond b. an ionic bond 6. What is electronegativity and how is it related to chemical bonding? Periodic Table Groups-homework(9.2) 1. Color-code the following groups on your periodic table: -alkali group -alkaline group - transitional group -halogen group -noble gases -inner transitional group 2. Add the staircase (separates metals/non-metals.) 3. Place an asterisk by each metalloid. 4. Non-metals groups: Which group is most reactive and which is most stable? Explain how you know this. 5. Metal groups: Which group is most reactive and which is most stable? Explain how you know this. 6. Give an example of two elements that will form an ionic bond to reach stability. 7. Give an example of two elements that will form a covalent bond to reach stability. 8. What does each period on the periodic table symbolize for an atom? 9. What period and group is Bromine in? 10. a. What is electronegativity? b. What is the trend in electronegativity across a period and down a group on the periodic table. Chemical Nomenclature • Naming and deriving the formula of compounds . Chem II-Period: 9.12.14 Graded: • Periodic Table Bell Ringer Objectives: • Chemical classification and nomenclature • Relationship between chemical bonding and organization of periodic table. Homework: • Chemical Nomenclature Packet(ionic/covalent) • Chemical Bonding Study Guide Chemical Nomenclature Chem II-Period: 9.16.14 Due: • Chemical Nomenclature Packet (ionic/covalent) Objectives: • Chemical classification and nomenclature(Acids/Bases) • Relationship between chemical bonding and organization of periodic table. Chemical Nomenclature Chem II-Period: 9.17.14 Due: • Chemical Nomenclature Packet (ionic/covalent) Objectives: • Chemical classification and nomenclature(Acids/Bases) • Relationship between chemical bonding and organization of periodic table. Homework: • Electronegativity Values and Chemical Bonding packet-Due Friday! (ppts. on my webpage) Chemical Nomenclature Nomenclature Gallery Walk Classify: Ionic or Covalent Chemical Formula Chemical Name Chem II-Period: 9.19.14 Infinite Campus: • Chemical Nomenclature Lab (10pts.) Objectives: • Chemical classification and nomenclature. • Relationship between chemical bonding and electronegativity. Homework: • Chemical Classification and Nomenclature-Quiz Chem II-Period: 9.22.14 Objectives: • Chemical classification and nomenclature. • Relationship between chemical bonding and electronegativity. • Drawing Chemical Structures-Lewis Dot Structures Homework: • Chemical Classification and Nomenclature-Quiz • Chemical Bonding/Structures Packet Chemical Nomenclature Bell Ringer Complete the table below for each compound. Chemical Name Classification (ionic, covalent, acid, base) Chemical Formula manganese (II) sulfate phosphorous trichloride carbonic acid BrCl5 Sr(OH)2 H3P Chemical Nomenclature Quiz: A Complete the table below for each compound. Chemical Name Classification (ionic, covalent, acid, base) Chemical Formula Phosphoric acid copper (I) sulfate Phosphorous tetrachloride Al(NO2)3 H2S SO Chemical Nomenclature Quiz:B Complete the table below for each compound. Chemical Name Classification (ionic, covalent, acid, base) Chemical Formula dinitrogen pentasulfide iron (III) acetate hydrofluoric acid H(NO3) Ba(NO3)2 SiCl4 Chemical Bonding and Electronegativity Electronegativity • Electronegativity: The degree of attraction one atom has towards another atom’s valence electron in a compound. • Determines the type of chemical bonds in a compound. (ionic, non-polar, and polar) O H H Electronegativity Values webassign.net Chemical Bonding : Electronegativity Difference • The eletronegativity (EN) difference is calculated by subtracting the electronegativity values of each atom within a single bond. Electronegativity Values • According to the eletronegativity table below, would metals or non-metals have a greater electronegativity value? • Explain how electronegativity values determine the type of ion (cation/anion) that a metal and non-metal would form in an ionic compound. webassign.net Electronegativity Values webassign.net Covalent Compounds: Polar vs.Non-Polar Bonds Chemical Bonding : Electronegativity Difference • The eletronegativity (EN) difference is calculated by subtracting the electronegativity values of each atom within a single bond. Chem II-Period: 9.23.14 Due: Chemical Bonding Table Worksheet Objectives: • Chemical classification and nomenclature(quiz). • Relationship between chemical bonding and electronegativity. • Drawing Chemical Structures-Lewis Dot Structures Homework: • Chemical Bonding/Structures Packet Chemical Bonding and Electronegativity Chemical Formula Classify Compound Chemical Name Bonds: Ionic, Polar, Non-polar (EN difference) CO2 C---O CI4 C----I MgF2 Mg---F N2 N----N FeO Fe---O Chem II-Period: 9.24.14 Infinite Campus: Chemical Cmpds. Quiz (12pts.) Due: Electronegativity and Chemical Bonds Objectives: • Relationship between chemical bonding and electronegativity. • Drawing Chemical Structures-Lewis Dot Structures Homework: • Chemical Bonding/Structures Packet Chem II-Period: 9.25.14 Infinite Campus: Chemical Cmpds. Quiz (12pts.) Due: Electronegativity and Chemical Bonds Objectives: • Relationship between chemical bonding and electronegativity. • Drawing Chemical Structures-Lewis Dot Structures Homework: • Chemical Bonding/Structures Packet Chemical Compounds Quiz Chemical Bonding : Electronegativity Difference Modifications to electronegativity difference table? Covalent Compounds: Polar vs.Non-Polar Bonds Ionic Bonding (Lewis Dot Transfer) Metal (Lewis Dot) Mg Non-metal (Lewis Dot) F Ionic Bonding (Lewis Dot Transfer) Molecular Structures: Lewis Dot Sharing Molecules CS2 CO2 N2 Lewis Dot Sharing Molecular Name Polar or NonPolar Bonds Drawing Molecular Structures 1.Draw Lewis Dot Structure for each element. 2.Calculate the total number of ve- for the molecule . 3.Share unpaired ve- with atoms. (covalent bonds) 4.Place lone pairs around appropriate atoms. 5.If an atom is not stable, then lone pairs can be used to reach maximum stability. 6.There are some exceptions to Octet Rule • Elements with d-orbitals • Molecules with an odd number of ve- (total) VSEPR Theory (Valence Shell Electron Pair Repulsion) liakatas.org Molecular Geometry • Shape of the molecule in 3-D space. • VSEPR Theory: -(Valence Shell Electron Pair Repulsion) -How bonds and lone pairs are arranged around atoms to minimize electron repulsion. Chem II-Period: 9.29.14 Objectives: • Chemical Compounds: classify/nomenclature-quiz • Predict chemical bonding using electronegativity. • Illustrate ionic and covalent bonding using Lewis Dot Structures. • Identify and model geometries of covalent compounds. (VSEPR theory) • Classify the type of bond between atoms in a molecule (sigma/pi). • Classify molecules as either polar or non-polar. • Predict the types of intermolecular forces that exist between molecules. Homework: Chemical Bonding Study Guide-test Fri. Molecular Structure Lab Purpose: • Model the geometrical shape of molecules using the VSEPR diagram. • Additional Molecules: • CH2Cl2 • CO • (NH4)+ • PCl5 • BF3 Chem II-Period: 9.30.14 Due: • Molecluar Structure Lab (pre-lab, L-D structures) Objectives: • Identify and model geometries of covalent compounds. (VSEPR theory) • Classify the type of bond between atoms in a molecule (sigma/pi). • Classify molecules as either polar or non-polar. • Predict the types of intermolecular forces that exist between molecules. Homework: Chemical Bonding Study Guide-test Fri. . Molecular Structure Lab Purpose: • Model the geometrical shape of molecules using the VSEPR diagram. • Additional Molecules: • CH2Cl2 • CO • (NH4)+ • PCl5 • BF3 Molecular Structures Chem II-Period: 10.01.14 Due: • Molecluar Structure Lab (classify bonds and polarity of molecule). Objectives: • Identify and model geometries of covalent compounds. (VSEPR theory) • Classify the type of bond between atoms in a molecule (sigma/pi). • Classify molecules as either polar or non-polar. • Predict the types of intermolecular forces that exist between molecules. Homework: Chemical Bonding Study Guide-test Fri. Molecular Structures Exceptions: • Sometimes lone pairs are used in bonding (coordinate bonds) • There are exceptions to the octet rule: (S, P, B, H, He) Molecular Orbitals • When ve- are shared between two atoms in a molecule, their atomic orbitals overlap. • The overlap of atomic orbitals between two atoms create molecular orbitals. • Each molecular orbital is also called a bonding orbital because it represents the sharing of two ve- between two atoms. • Two types of bonding orbitals: Sigma and Pi Bonding Orbitals chemistryland.com Molecular Orbitals wikis.lawrence.edu Polar Molecules • What are polar molecules? Polar Molecules • Polar molecules: One end of the molecule is partially positive and the other end is partially negative. • Also called dipole molecules. • Which molecules did you predict from the lab were polar molecules? Polar Molecules Polar or Non-Polar Molecules? P 3dchem.com F F F en.wikipedia.org ieshermanosbilingual.blogspot.com Chem II-Period: 10.02.14 Due: • Molecular Structure Lab (classify bonds and polarity of molecule). Objectives: • Identify and model geometries of covalent compounds. (VSEPR theory) • Classify the type of bond between atoms in a molecule (sigma/pi). • Classify molecules as either polar or non-polar. • Predict the types of intermolecular forces that exist between molecules. Homework: Chemical Bonding Study Guide-test Fri. Molecular Structures Molecules SiH4 CF4 NBr3 CS2 Molecular Structure Geometry/ Bond Angle Sigma/Pi Bonds Polar/Non Polar/Non-Polar Polar Bonds Molecule Molecular Structures Molecules SiH4 CF4 NBr3 CS2 Molecular Structure Geometry/ Bond Angle Sigma/Pi Bonds Polar/Non Polar/Non-Polar Polar Bonds Molecule Intermolecular Forces: Classes • Identify and define the types of intermolecular forces that can exist between molecules in the liquid and solid state. • Give an example of a molecule that applies each type of intermolecular force when in the solid and liquid state. Intermolecular Forces Forces between molecules in the solid, liquid, and gaseous state. itl.chem.ufl.edu Types of Intermolecular Forces 1.Dipole Forces : • Attraction b/w polar molecules • Partial charges oppositely attract Prentice Hall: Chemistry Types of Intermolecular Forces Hydrogen Bonds : • Very strong dipole force. Pre-resquiste for H bonding to occur: • H must be covalently bonded to a very electronegative element (N, O, F). Prentice Hall: Chemistry Types of Intermolecular Forces 3. London Dispersion Forces: Caused by randomn motion of electrons Occur in non-polar and polar molecules. Temporary dipole forces. I I • • • • elmhurst.edu Intermolecular Forces Rank the intermolecular forces from weakest to strongest? itl.chem.ufl.edu Intermolecular Forces Molecular Formula Polar or Nonpolar Molecule Intermolecular Forces CS2 Non-polar molecule because the bonds are non-polar; symmetrical. Van der Waals or London Dispersion forces because the molecule is non-polar. Polar molecule because bonds are polar, central atoms has lone pairs, and the geometry is asymetrical. Hydrogen Bonding, strong dipole forces because hydrogen is bonded to a very electronegative element, N. Polar molecule because bonds are polar and the central atom has to sets of lone pairs. Also the molecule is asymetrical. Dipole Bonding, because the molecule is polar, meaning there is a partial charges that exist in the molecule. NH3 OCl2