Chemistry Chapter 12 Study Guide: Bonding & Molecular Shape

Chemistry
Chapter 12 Test Study Guide
Know the following:
1.
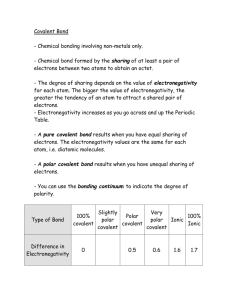
Be able to distinguish between a pure covalent, a polar covalent and an ionic bond by examining the bond between 2 atoms.
2.
Use the electronegativity chart to predict which element (given 2 elements) has a greater electronegativity value.
3.
Distinguish lone pairs from bonding pairs of electrons
4.
Using the electronegativity chart, determine the electronegativity difference between 2 bonding atoms, and classify the bond as pure covalent, polar covalent or ionic. (Hint: > 1.7 is ionic, < 1.7 is polar covalent, 0 electronegativity difference = pure covalent)
5.
Write out the Lewis structure for molecules and polyatomic ions.
6.
List a molecule as polar or non-polar based upon molecular shape.
7.
Describe what happens to atoms that develop ionic bonds.
8.
Describe both ionic and covalent bonds.
9.
Draw electron dot diagrams for representative elements
10.
Draw resonance structures
11.
Based on a chemical formula, predict the shape of a molecule as linear, trigonal planar, tetrahedral, trigonal pyramidal or bent.
There are 40 questions on the test