9.4-9.5 Review
advertisement

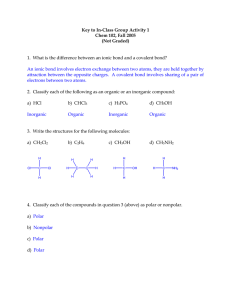
Name: _______________________ 9.4-9.5 Review 1. What is the VSEPR model? 2. What are the bond angles in a molecule with a tetrahedral shape? 3. What is hybridization? 4. Compare the molecules PF3 and PF5. What is the molecular shape of each of the two molecules? Why is the shape different? 5. Make a table that contains the Lewis structure, molecular shape and bond angle for the following molecules: CS2, CH2O, H2Se, CCl2F2, and NCl3. 6. Define electronegativity. 7. How is electronegativity difference used in determining the type of bond that occurs between two atoms? Name: _______________________ 8. Describe a polar covalent bond. 9. What is a polar molecule? 10. List 3 properties of a covalent compound. 11. Rank the 3 types of intermolecular (Vander Waals) forces from weakest to strongest. 12. Predict the type of bond that will form between the following atoms. a. H and S b. C and H c. Na and S Change in EN Bond Type 0-0.5 0.5-1.7 > 1.7 Nonpolar Polar (covalent) Ionic 13. Draw the Lewis structure for the SF4 and SF6 molecules and determine if each molecule is polar or nonpolar.