3.07 Lab Worksheet

3.07 Review and Lab Worksheet~ 35 Points
1.
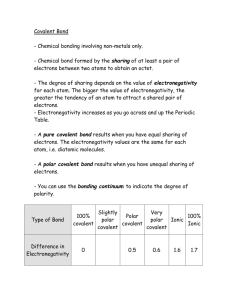
Determine the electronegativity between the atoms of each molecule.
Use the absolute value of one elements electronegativity minus the other elements electronegativity to determine the difference between the two.
2.
Identify the bond as either ionic or covalent.
Is the bond between a metal and non-metal (ionic) or two non-metals (covalent)?
3.
State whether the molecule is polar or non polar.
Remember~ If the difference in electronegativity for the atoms in a bond is greater than 0.4, we consider the bond polar. If the difference in electronegativity is less than 0.4, the bond is nonpolar.
4. Identify the structure as having hydrogen bonding, dipole-dipole moments or London dispersion forces
(LDF).
Ion-Dipole London Dispersion
Experienced by all molecules and particles
Dipole-Dipole
Experienced by all polar molecules
Hydrogen Bonding
Strongest intermolecular force
Ions interacting with polar molecules in solutions
Weakest intermolecular force
Ne, O2, and CO2
Electrostaticattraction between positive and negative poles
H2S and SO2
A particularly strong dipole-dipole force
HF, NH3, and H2O
Stronger as the charge on ion increases
NaCl + H2O
Compound
H2O
NH3
CH4
CO2
Electronegativity
Show work!
Ionic/Covalent Polar/Non HB, D-D or LDF