Chemistry Test 3: Chemical Bonding
advertisement

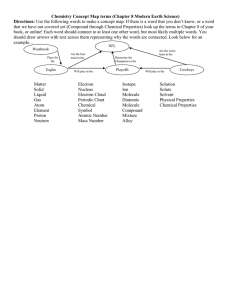
Chemistry Test 3: Chemical Bonding While previous tests have had more to do with ideas, this unit is based solely on DOING chemistry. This is a break from the way we have done things; later tests will be a combination of “doing” and “thinking” about chemistry. That being said, here is what you will be expected to do for tomorrow’s test: Given one-half of a chemical reaction, you should be able to: a. Complete the equation.* b. Balance the equation. c. Name all the compounds in the equation. d. Determine the types of bond found in each compound of the equation. e. Determine the Lewis Dot Structure/Bonding and shape of each molecule in the equation. f. Determine, if applicable, the polarity or non-polarity of each molecule in the equation. Some of the tools you can use: A copy (I’ll give it to you) of p 413 in your text; your periodic table, and maybe(?) a calculator. The most important thing is your brain, because you are going to have to think through thing! Some things you will need to know that might help you: Definition of Ionic and Covalent Bonds. The Polyatomic Ions. The Electronegativity Number Line. Greek Numerical Prefixes (for naming WHICH type of compound?). You may not have any of these items written down for your test, though. Good Luck!