Discussion: Lewis Structures and VSEPR Theory
advertisement

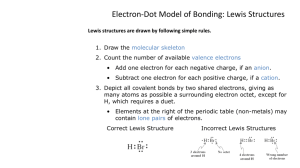
Discussion: Lewis Structures and VSEPR Theory OBJECTIVE: Students Will Understand Why Elements Form Bonds With Other Elements What are valence electrons? Electrons in the highest energy level Elements in the same group on the periodic table have the same number A valence of 8 electrons is called a octet Atoms try to get a valence of 8 electrons Not all elements try to get a valence of eight electrons Hydrogen needs only two. Beryllium needs on four Boron needs only six. However, most elements like to have eight electrons in their valence shell. How the Valence Electrons Fit Around Group #1 Atoms Valence Electrons for Group #2 Valence for the rest How Atoms Gain an Octet by Losing Electrons Calcium atom loses two electrons to achieve an octet How Atoms Gain an Octet by Gaining Electrons Chlorine has seven electrons. It gains one electron to make eight. The addition of an electron make the ion negative. Nobel Elements already have an octet. Neon for example has 10 electrons Notice it already has an octet. That is why it doesn't react with other elements Ions almost always have an octet Na+ ion for example, has 10 electrons just like Neon 2 8 Na+ | | lost 1 2 8 O2| | gained 2 2 8 Ne | | Atoms try to get this electron configuration AlCl3 Draw on board PCl3 Count all the valance electrons Determine the central atom. Connect the ligands to the central atom. VSEPR Theory Valence Shell Electron Pair Repulsion The VSEPR Theory Explains the geometric shape of a molecule based solely on the repulsion of electron pairs around the central atom. Geometric Shapes Linear Planer Triangular Tetrahedral Polar molecules Molecules that are unbalanced are polar. Planer Triangular Draw SO2 Total Electrons = 18 Central Atom = S Connect the two oxygen atoms to the central atom. Subtract 2 for each bond. Put six around the O. Put the extra around the central atom. Make the legends double share until all have octets. The Shape of SO2 Notice there are 3 pairs of electrons around the central atom. The Shape of H2O Draw the Lewis structure for Water NH3 Draw the Lewis structure for ammonia CH4 Draw the Lewis structure for methane