CH 115 Fall 2014Worksheet 9 What is the formula for calculating
advertisement

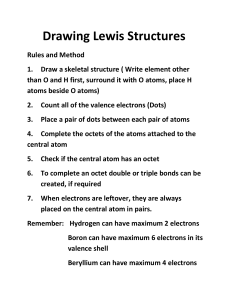
CH 115 Fall 2014 Worksheet 9 1. What is the formula for calculating formal charge? Why do we calculate formal charge? Formal charge = (number of valence electrons) – (number of lone pair electrons) – (1/2* number of bonding electrons) OR (number of bonds) We calculate formal charge in order to determine which of the Lewis structures we have drawn for a compound (if multiple are possible) is the best. Remember that 0 is the best formal charge for any atom to have. Negative numbers are better than positive numbers – formal charge is better when the negative charge is on the most electronegative of the atoms. The formal charge of each atom must add up to the total charge on the compound. 2. Draw the Lewis structure(s) for each of the following compounds. Include all resonance structures if applicable and calculate formal charge for each of the atoms. a). CH2CHF b). H2NCOCH3 c). O3 d). NH4+