Electrons & Ions: Note-Taking Worksheet for Chemistry
advertisement

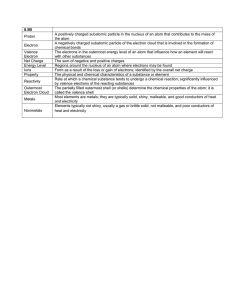
Focused Note Taking College and Career Ready NAME: KNOW: Know the properties of electrons related to orbitals & energy levels KNOW: Know the properties of ions and how they are formed __________________________________________________________________________ DO: Be able find an element’s number of valence electrons using the Periodic Table DO: Be able draw and element’s Bohr model and Lewis Dot Structure __________________________________________________________________________ ESSENTIAL QUESTION: 1) How do the interactions between subatomic particles and their arrangement affect the properties of an element? 2) How does the arrangement of elements and the energy of those atoms affect the properties of a material? Mrs. Stephenson _________________________________ CLASS/BLOCK: _________________________________ DATE: _________________________________ TOPIC: ELECTRONS Sec. 3.6 – 3.8 (pgs. 104 – 118) _________________________________ QUESTIONS: NOTES: What is the mass and charge of an electron? Where would one find electrons in an atom? Explain the concept of orbitals in an atom: Why are specific electrons located in the specific level and orbital that they occupy and nowhere else in the atom? Define a Valence Electron: (fill in: ) How does the number of valence electrons affect the arrangement of the Periodic Table? What is an Ion? What is the difference between a Cation and an Anion? Define a Lewis Dot Diagram, and tell what they are used for: Why does Sodium give up an electron when it bonds with Chlorine to form salt? (fill in: What is the goal of every atom? Summary: )