209BHJAN04 - Kuwait University
advertisement
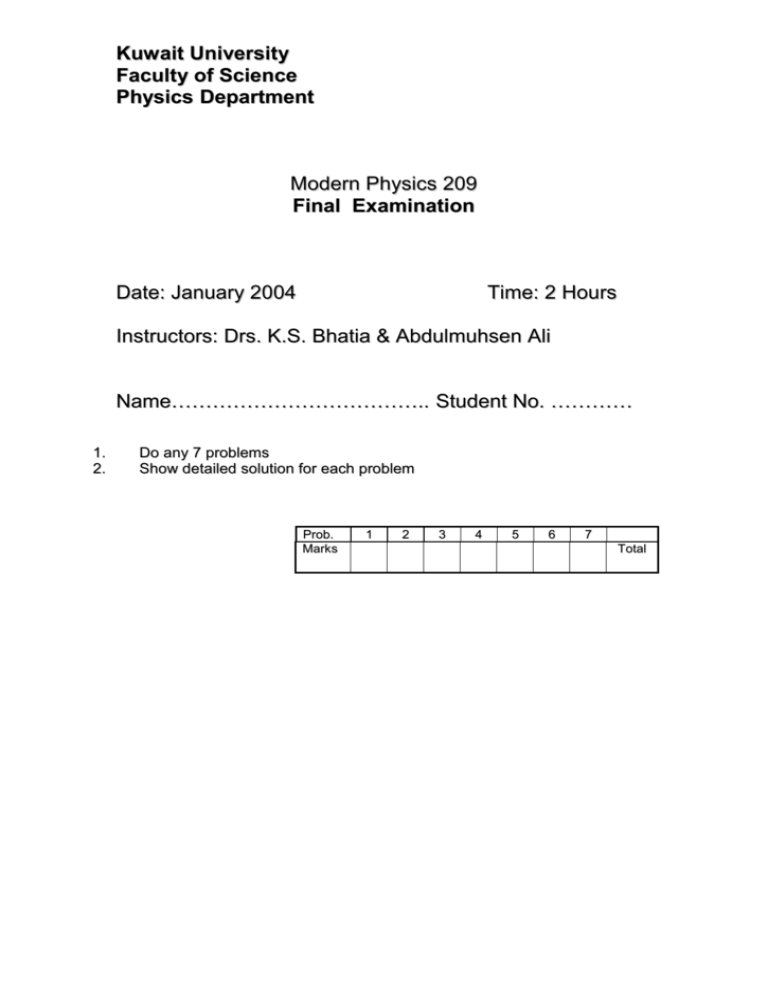
Kuwait University Faculty of Science Physics Department Modern Physics 209 Final Examination Date: January 2004 Time: 2 Hours Instructors: Drs. K.S. Bhatia & Abdulmuhsen Ali Name……………………………….. Student No. ………… 1. 2. Do any 7 problems Show detailed solution for each problem Prob. Marks 1 2 3 4 5 6 7 Total 1. Prove the following equations: Total energy E of mass m is E = mc2 c is the velocity of light. momentum p = 1 E - moc 2 c 2. A and B are twin brothers of 10 year age. A makes a round trip to a galaxy 100 Light years away with a speed of 0.85 C, while B remains on the earth. W hat is the age difference between A and B when they meet again? 3. X-rays of = 0.2 nm are scattered from carbon block. Binding energy of the electron in carbon atom is 4 eV. Calculate the a) b) Energy of the scattered photon at 45. The corresponding angle and momentum of the scattered electron. 4. A laser light of 300 nm and intensity 1 mw/cm2 falls on 1cm2 of a photosensitive surface ( workfunction = 2 eV). Calculate the velocity of emitted electrons and the current generated by the photo surface. (Assume each photon releases one photoelectron). 5. An electron trapped in an infinite potential well of length 0.1 nm transfers from 3rd energy state to 1st energy state and it radiates the energy in the form of electromagnetic wave. Calculate the wavelength of the radiation. 6. A hydrogen atom in an excited state emits wavelength of = 102.5 nm when the electron returns to the ground state. Calculate the principle quantum number n for the excited state. 7. A hydrogen atom is 5.3 10-11 m in radius. Use uncertainty principle to estimate minimum energy an electron can have in this atom. W hat is the actual value for lowest energy of an electron in hydrogen atom. 8. 9. Calculate all the angles of L vector with the z vector for the orbital quantum Number = 2. Show that the radial probability density of the 1s level has its maximum Value at r = ao (Hint: Radial wavefunction for 1s = ( 2/ao3/2 ) e-r/a and radial probability is given by P(r) = r2 | Rn (r) | 2