Exam Review - Chemistry(CHEMISTRY )modified
advertisement

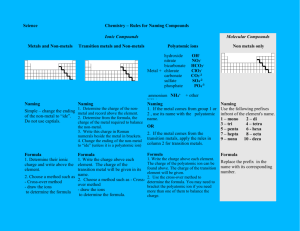
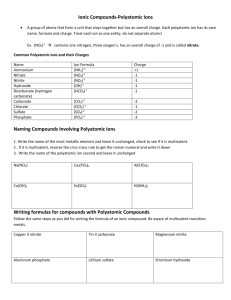
Chemistry Exam Review Binary Ionic Compounds (metal ion from groups 1 to3) • contain only two atoms – one metal + one non-metal Naming: 1) 2) 3) name the metal. then name non-metal and change ending to “ide” check for multivalent metals (use roman numerals) ** see next slide for details** Formula: 1) 2) 3) 4) write symbols (metal first, non-metal second) write charges above criss-cross numbers simplify if possible (reduce to lowest terms) Binary Ionic Compounds (Multivalent) • contain only two atoms one metal (iron, cobalt, nickel, copper, gold, mercury, tin or lead) + one non-metal Naming: 1) name the metal 2) reverse the criss-cross rule by putting the subscripts above each element symbol where they originally came from 3) check to see if the charge of the non-metal element matches its charge on the periodic table… a) if it does, change the charge of the METAL to Roman numerals and write it in brackets BEHIND the name of the metal b) if it does not, multiply BOTH the charge of the metal and non-metal by what the non-metal’s charge should be (according to the periodic table) and change the charge of the METAL to Roman numerals and write it in brackets BEHIND the name of the metal 4) then name non-metal and change ending to “ide” Polyatomic compounds • more than two atoms (count the number of capital letters) – metal ion + polyatomic negative ion (most are negative) OR – polyatomic positive ion + non-metal ion OR – polyatomic positive ion + polyatomic negative ion Naming: 1) 2) 3) name positive ion then name negative ion – polyatomic ion names do not change, only change nonmetal ion’s name if it is an element from groups 5 to 7 check for multivalent metals (use Roman numerals) Formula: 1) 2) 3) 4) 5) write symbols write charges criss-cross numbers when placing a subscript BEHIND a polyatomic ion, brackets must be placed around the polyatomic ion formula simplify if possible – remember ** polyatomic ion formulas cannot be changed! ** Binary Acids • two 2 atoms – starts with H+ ion + non-metal from groups 5 to 7 Naming: 1) H becomes "hydro" 2) name non-metal and change ending to "ic" 3) add the word “acid” to the end of the name Formula: 1) 2) 3) 4) write the symbols for each element write charges criss-cross numbers add (aq) to the end of the formula Examples: • Give the formula: hydrofluoric acid • Give the name: HCl(aq) hydriodic acid HBr(aq) Oxyacids • more than 2 atoms – always starts with H + polyatomic ion containing oxygen is the second ion • Naming: 1) 2) 3) • name the polyatomic ion If ending of the polyatomic is "ate" change ending to "ic” and add the word “acid" If ending of the polyatomic is "ite" change ending to "ous” and add the word “acid“ Formula: 1) write symbols 2) write charges 3) criss-cross numbers 4) add (aq) to the end of the formula Examples: • Give the formula: nitrous acid – HNO3(aq) • Give the name: H2SO4(aq) – sulfuric acid chloric acid - HClO2(aq) H3PO3(aq) – phosphorous acid Molecular compounds • two atoms – only non-metals • Naming: 1) Name first non-metal using prefixes (not “mono”) 2) Name the second non-metal using all prefixes 3) Change ending to “ide” • Formula 1) Write symbols 2) Prefix tells how many of each atom Balancing Chemical Equations 1) Cannot change any of the subscripts 2) Only add number to the front of the molecules 3) Leave the simplest molecules or lone atoms until last 4) Work on the most complicated molecules first Word/Chemical Equations • Need same number and type of atoms on reactant and product side of equation Word Equation Skeleton Equation Balanced Equation Types of Reactions 1) Synthesis reaction • A + B AB 2) Decomposition reaction • AB A + B 3) Single displacement reaction • AB + C AC + B or A + BC 4) Double displacement reaction • AB + CD AD + CB Predicting Products • Need same type and number of atoms on reactant side and product side • Identify which type of reaction it is – Switch atoms accordingly • Balance equation Examples • Na + O2 • Na2S + Al • FeS • Cl2 + NaBr • NaCl + Cu2SO4 • C4H6 + O2 Acids/Bases/PH Acid Base Neutral Change in pH from 1 to 2 pH scale Change in the number of H+ ions _______ times more ________ _______ times less _________ from 2 to 5 _______ times more ________ _______ times less _________ from 12 to 8 _______ times more ________ _______ times less _________ Solution A ACID Solution C NEUTRAL Solution B BASE