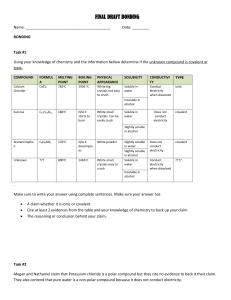
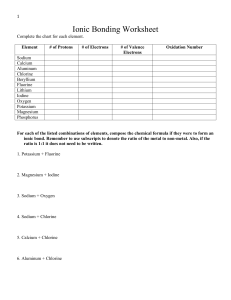
Document
advertisement
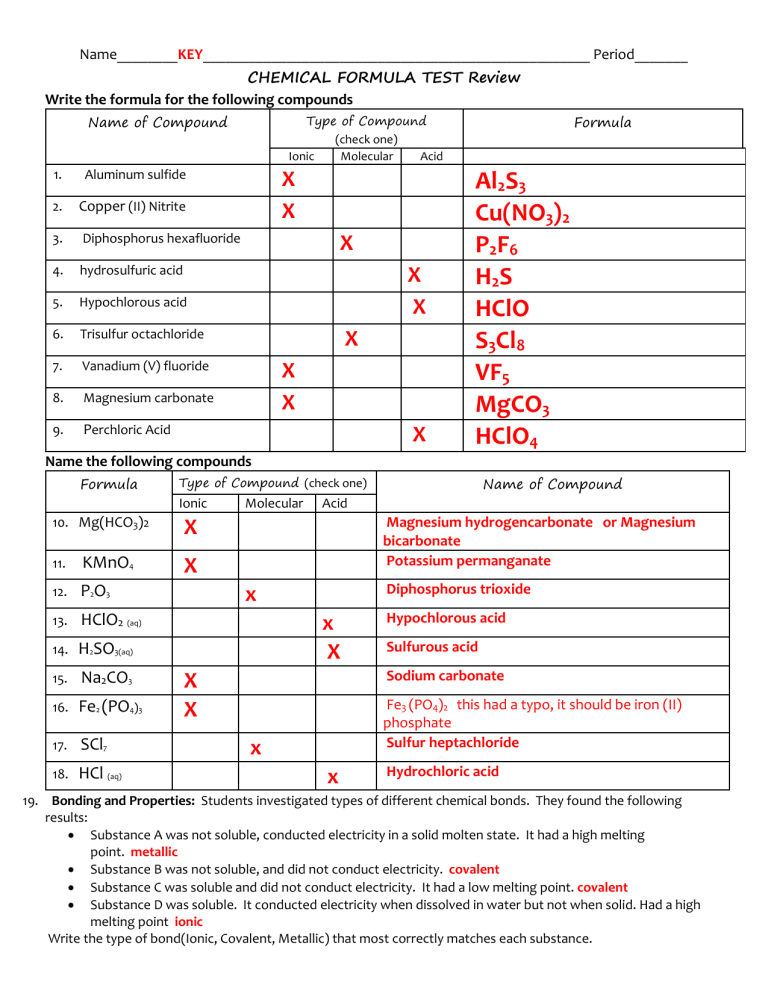
Name________KEY___________________________________________________ Period_______ CHEMICAL FORMULA TEST Review Write the formula for the following compounds Name of Compound 1. Aluminum sulfide 2. Copper (II) Nitrite 3. Diphosphorus hexafluoride 4. hydrosulfuric acid 5. Hypochlorous acid 6. Trisulfur octachloride 7. Vanadium (V) fluoride 8. Magnesium carbonate 9. Perchloric Acid Formula Type of Compound (check one) Ionic Molecular Acid X X X X X X X X X Al2S3 Cu(NO3)2 P 2 F6 H2S HClO S3Cl8 VF5 MgCO3 HClO4 Name the following compounds Formula Ionic 10. Mg(HCO3)2 11. KMnO4 12. P2O3 13. HClO2 (aq) 14. H2SO3(aq) 15. Na2CO3 16. Fe2 (PO4)3 17. SCl7 18. HCl (aq) Name of Compound Type of Compound (check one) Molecular Acid Magnesium hydrogencarbonate or Magnesium bicarbonate Potassium permanganate X X Diphosphorus trioxide x x X Hypochlorous acid Sulfurous acid Sodium carbonate X X Fe3 (PO4)2 this had a typo, it should be iron (II) phosphate Sulfur heptachloride x x Hydrochloric acid 19. Bonding and Properties: Students investigated types of different chemical bonds. They found the following results: Substance A was not soluble, conducted electricity in a solid molten state. It had a high melting point. metallic Substance B was not soluble, and did not conduct electricity. covalent Substance C was soluble and did not conduct electricity. It had a low melting point. covalent Substance D was soluble. It conducted electricity when dissolved in water but not when solid. Had a high melting point ionic Write the type of bond(Ionic, Covalent, Metallic) that most correctly matches each substance. 20. Write the electron dot diagrams for the following molecules and name the shape of the molecule. COMPOUND LEWIS STRUCTURE SHAPE Tetrahedral a. PH3 pyramid Bent c. Nonpolar CCl4 Trigonal b. POLARITY H2S Polar polar