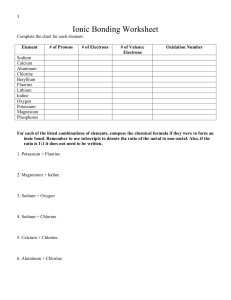
1 Ionic Bonding Worksheet Complete the chart for each element. Element # of Protons # of Electrons # of Valence Electrons Oxidation Number Sodium Calcium Aluminum Chlorine Beryllium Fluorine Lithium Iodine Oxygen Potassium Magnesium Phosphorus For each of the listed combinations of elements, compose the chemical formula if they were to form an ionic bond. Remember to use subscripts to denote the ratio of the metal to non-metal. Also, if the ratio is 1:1 it does not need to be written. 1. Potassium + Fluorine 2. Magnesium + Iodine 3. Sodium + Oxygen 4. Sodium + Chlorine 5. Calcium + Chlorine 6. Aluminum + Chlorine 2 Compound Names and Formulas For the list on the left, name the compound. For the list on the right, give the chemical formula that corresponds to the name Name Formula 1) NaF 13) potassium fluoride 2) K2CO3 14) ammonium sulfate 3) MgCl2 15) magnesium iodide 4) Be(OH)2 16) copper (II) sulfite 5) SrS 17) aluminum phosphate 6) Cu2S 18) lead (II) nitrite 7) ZnI2 19) cobalt (II) selenide 8) Ca3(PO4)2 20) silver cyanide 9) NH4I 21) copper (II) carbonate 10) Mn(NO3)3 22) iron (II) oxide 11) FePO4 23) lithium cyanide 12) CoCO3 24) lead (IV) sulfite 3 Naming Acids and Bases Name the following acids and bases: 1) NaOH _______________________________________ 2) H2SO3 _______________________________________ 3) H2S _______________________________________ 4) H3PO4 _______________________________________ 5) NH3 _______________________________________ 6) HCN _______________________________________ 7) Ca(OH)2 _______________________________________ 8) Fe(OH)3 _______________________________________ 9) H3P_______________________________________ Write the formulas of the following acids and bases: 10) hydrofluoric acid _______________________________________ 11) hydroselenic acid _______________________________________ 12) carbonic acid _______________________________________ 13) lithium hydroxide _______________________________________ 14) nitrous acid _______________________________________ 15) cobalt (II) hydroxide _______________________________________ 16) sulfuric acid _______________________________________ 17) beryllium hydroxide _______________________________________ 18) hydrobromic acid _______________________________________ 4 Covalent Practice 1. Describe how a covalent bond forms. 2. Covalent bonds form between what kinds of elements? 3. For each element, draw the Lewis dots diagram (based on valence electrons). a) N d) P b) Cl e) Br c) Se f) I 4. For each compound, draw the Lewis dot diagram. a) CH4 d) Br2 b) PH3 e) N2 c) H2O f) BF3 d) HCN h) SiO2 5 Name the following covalent molecules: N2 O P4O10 SO3 CO2 N2O5 SCl2 OCl SO2 CS2 OF2 SiCl4 P2S3 SeCl2 OBr2 Give the formula for the following covalent molecules. Nitrogen trisulfide Dioxygen monochloride Sulfur dioxide Carbon tetrachloride Nitrogen pentoxide Sulfur hexafluoride Silicon heptasulfide Boron tribromide Carbon monoxide Carbon dioxide Phosphorus trichloride Oxygen difluoride 6 Types of Chemical Bonds a. Classify the following as ionic (metal + nonmetal) or covalent (nonmetal + nonmetal). b. Determine the charge for each element or polyatomic ion in each ionic compound. 1. CaCl2 11. MgO 2. CO2 12. NH4Cl 3. H2O 13. HCl 4. BaSO4 14. KI 5. K2O 15. NaOH 6. NaF 16. NO2 7. Na2CO3 17. AlPO4 8. CH4 18. FeCl3 9. SO3 19. P2O5 10. LiBr 20. N2O3 7 Naming and Formula Writing You must name the following. First you must determine whether the compound is ionic or covalent. Place an I or C in the first blank for each item, then name it correctly. _____ NaCl ______________________ _____ Ca(CN)2 ______________________ _____ Mg3(PO4)2 __________________ _____ N2O5 ________________________ _____ CrO3 _______________________ _____ V3(PO4)4 _____________________ _____ CBr4 ______________________ _____ PCl6 _________________________ _____Mn2O7 _____________________ _____ SiO2 _________________________ _____ CO ______________________ _____ K2O _________________________ _____ N3O7 ______________________ _____ AlBr3 ________________________ ____ Nitrogen tetroxide ____________ ____ Sulfur trioxide __________________ ____ Aluminum chloride ___________ ____Tetraphosphorus decoxide __________ ____ Iron (III) perchlorate __________ ____ Copper (II) hydroxide_____________ ____ Carbon dioxide ______________ ____ Tetraphosphorus trisulfide__________ _____ Chromium (VI) carbonate___________ ____ Calcium dichromate ______________ _____ Sulfur hexoxide ______________ ____ Potassium phosphate ______________ 8 Molecular Shapes Formula HBr BeF2 BCl3 CH4 NH3 H2Se HCN PF3 CBr4 Lewis Diagram Pairs of e- (# Bonding Pairs, # Lone Pairs) Shape Angle Between Bonds 9 Chapter 9 Study Guide: Naming & Writing Formulas 1. When group 3A elements form ions do they lose or gain electrons? How many? 2. When naming a transition metal ion what does the Roman numeral mean? 3. What is the ion that Ca usually forms? 4. What is the charge on the Mn in each of the following? MnS MnBr2 Mn2O3 MnPO4 5. Ionic compounds are formed by what? 6. Write the following in the correct formula: Ca2+ F1Na+ Cl- Ba2+ O2Pb4+ O2- 7. What is the charge on the Pb in each of the following? PbO PbCl4 Pb2O Pb2S 8. Write the correct formula for Magnesium sulfite. 9. Give the correct name of the following formulas: (NH4)2S LiCO3 FePO4 MgCrO4 10. Covalent compounds are formed by what? 11. What is the ending for all binary compounds? 12. In covalent compound names, how is the number of atoms of each element indicated? 14. What is the name of CoCl2 15. Name the following: NO3 SO4 NO2 16. Which is correct for Mg3N2? a) trimagnesium dinitride b) Magnesium nitride c) Magnesium(II) nitride 10 17. Give the formula for the following: a) Flourine b) Oxygen c) sulfur trioxide d) sodium sulfite e) calcium hydroxide f) iron(III) flouride g) Lead(II) carbonate h) mercury(II) sulfide j) copper(II) sulfate k) magnesium oxide l) Barium hydrogen sulfate m) gold(I) chloride n) ammonium thiocyanate 18. Give the name of the following: a) H2 b) PO4 c) SI9 d) MgSO3 e) Sn(SO4)2 f) Na3PO4 g) Au2S3 h) Ca(OH)2 i) LiMnO4 k) MnS l) CuSO4 m) Li2Cr2O7 n) Ag2CO3