Study Guide for Bonding and Flame Test Test
advertisement

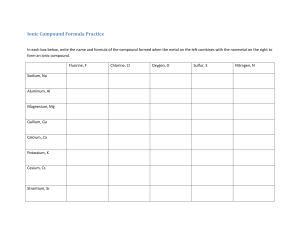
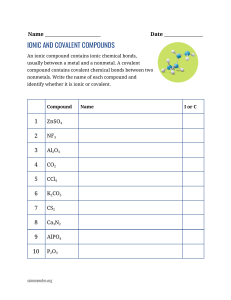
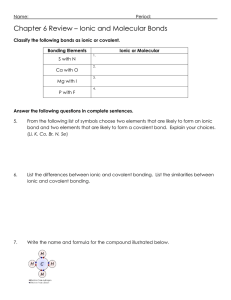
Study Guide For Chemistry Quiz on Bonding and Flame Test 1) What is an ionic compound? 2) What are 4 properties of ionic compounds? - Be able to identify an ionic compound given a set of properties 4) What is a covalent compound? 5) What are 4 properties of covalent compounds? - Be able to identify a covalent compound given a set of properties 6) Which group on the periodic table does not form bonds (because they have a complete outer shell? 7) Define compound and provide at least 2 examples of compounds 8) Define the Ground State for an element. 9) Draw the electron configuration for Magnesium. How many shells does Mg have? 10) Define the Excited State for an element. 11) How does an element produce photons (list the order of steps, use at least 3 steps)? 12) Why does each element give off a different color when heated? 13) Will both metals and non-metals act the same in a flame test? EXPLAIN using a discussion of electrons. 14) How do fireworks make different colors? 15) List the order of colors from longest wavelength to shortest. And then label which color has the highest energy, and which color has the lowest energy. 16) In a flame test, does strontium or lead give off higher energy photons? Explain your answer.