Bonding CER for CCSS

FINAL DRAFT BONDING
Name: _________________________________________
BONDING
Task #1
Date: ________
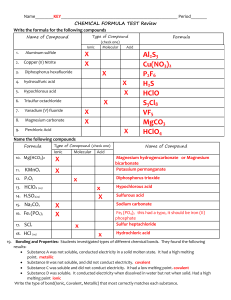
Using your knowledge of chemistry and the information bellow determine if the unknown compound is covalent or ionic.
COMPOUND FORMUL
Calcium
Chloride
A
CaCl
2
MELTING
POINT
782 o C
Sucrose C
12
H
22
O
11
186 o C
BOILING
POINT
1936 o C
N/A it starts to burn
PHYSICAL
APPEARANCE
White big crystals not easy to crush
SOLUBILITY CONDUCTIVI
Soluble in water
TY
Conduct electricity when dissolved
Insoluble in alcohol
TYPE ionic
White small crystals. Can be easily crush
Soluble in water
Slightly soluble in alcohol
Does not conduct electricity covalent
Acetaminophe n
Unknown
C
8
H
9
NO
2
170 o C
??? 800 o C
N/A it decompos es
White powder
1465 o C White small crystals easy to crush
Slightly soluble in water
Slightly soluble in alcohol
Soluble in water
Does not conduct electricity
Conduct electricity when dissolved covalent
????
Insoluble in alcohol
.
Make sure to write your answer using complete sentences. Make sure your answer has
A claim whether it is ionic or covalent
Cite at least 2 evidences from the table and your knowledge of chemistry to back up your claim
The reasoning or conclusion behind your claim.
Task #2
Megan and Nathaniel claim that Potassium chloride is a polar compound but they cite no evidence to back it their claim.
They also contend that pure water is a non-polar compound because it does not conduct electricity.
Prove or disprove their claim by citing physical, chemical or electro-negativity difference evidences. Make sure to write your answer using full sentence structure.
Task #3
What type of compound you get when you burn solid magnesium in the present of oxygen?
1) Write a balance chemical equation to represent chemical the reaction.
2) Make a claim that includes whether the compound formed in the product is polar, ionic or non-polar and why.
The periodic table is a great toll to chemists. It not only contains the elements but provides you information as to their properties. Recently you learned how to use the period table to predict the type of compounds or the type of bonds that you can form.
For example
Ionic Compound: Na + Cl NaCl
Covalent Compound: S + O2 SO2
Non-polar Cl + Cl Cl2
Metalic: Fe + Fe 2Fe or
Zn + Fe a galvanized iron that would not rust
Base on the information above provided, the reference table and your knowledge of Chemical bonding can you provide a easy way to remember how to identify the type of bond when we look at the elements forming the compound.
Metalic:
Ionic:
Covalent Polar
Covalent Nonpolar: