Chemical Bonds, Formulas, Naming Compounds, and Chemical
advertisement

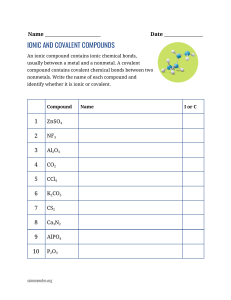
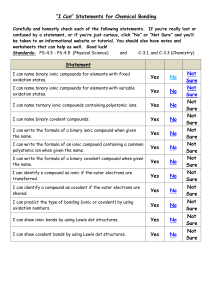
Chemical Bonds, Formulas, Naming Compounds, and Chemical Reactions Name: ______________________________________ Vocabulary: Cation Covalent Bond Anion Subscript Compound Chemical Formula Ionic Bond Oxidation Number Polyatomic Law of Conservation of Mass What are the parts of a Chemical Equation and what do they mean? CaCO3(s) + 2 HCL(aq) CaCl2(aq) + H2O(l) + CO2(g) Product Reactant Produces/Yields Coefficient Subscript Solid Liquid Gas Aqueous What are the steps to name an ionic compound? What are the names and oxidation numbers for the following found in polyatomic compounds? NO2 NO3 OH PO4 CO3 SO4 List the steps to name a covalent compound. List the prefixes used to identify the number of atoms of an element in a chemical formula when it is covalent (Non-metal – Nonmetal). One Six Two Seven Three Eight Four Nine Five Ten Practice Writing Formulas and Naming Compounds (both Ionic and Covalent): Formula or Formula or Compound Name Type of Bond Compound Name 1. Magnesium Sulfide ___________________ ________________________ 2. K2O ___________________ ________________________ 3. CO ___________________ ________________________ 4. Dinitrogen Monoxide ___________________ ________________________ 5. MnBr2 ___________________ ________________________ 6. Calcium Hydroxide ___________________ ________________________ 7. NaNO3 ___________________ ________________________ 8. MgCl2 ___________________ ________________________ [Type text] Directions: Use a periodic table, complete the table below: Element Total # of Protons Total # of Electrons Oxidation Number (Do not forget the required sign +/-) # of Valence Electrons Cesium Beryllium Calcium Iodine List and explain the 5 types of Chemical Reactions. 1. 2. 3. 4. 5. Describe evidence that would tell you a chemical reaction has occurred. How do the following affect the Rate of a Chemical Reaction? Surface Area Concentration Catalyst Temperature Inhibitor