Characteristics of Acids: Table K
advertisement
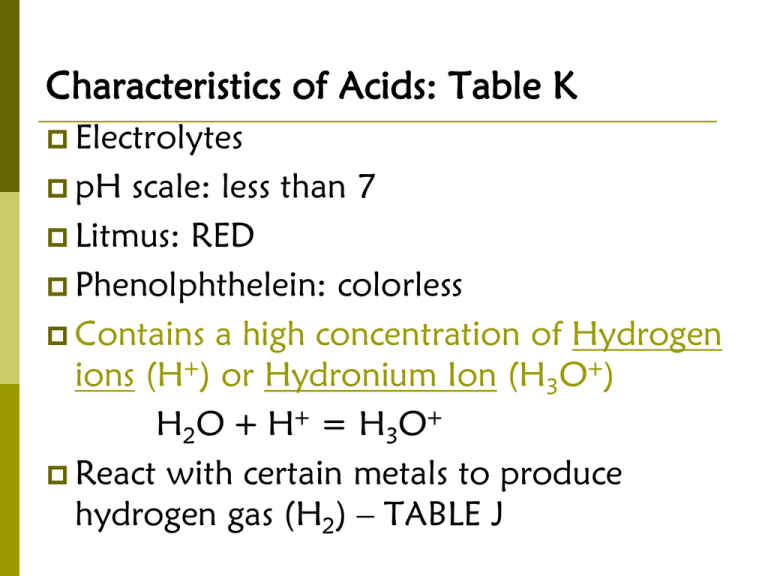
Characteristics of Acids: Table K Electrolytes pH scale: less than 7 Litmus: RED Phenolphthelein: colorless Contains a high concentration of Hydrogen ions (H+) or Hydronium Ion (H3O+) H2O + H+ = H3O+ React with certain metals to produce hydrogen gas (H2) – TABLE J Metal must be higher than H2 to react Characteristics of Bases: Table L Electrolytes pH scale: greater than 7 Litmus: BLUE Phenolphthelein: pink Contains a high concentration of Hydroxide ions (OH-) Tastes bitter; feels slippery/soapy Characteristics of Salts An ionic compound that has positive ions other than hydrogen (H+) and negative ions other than hydroxide (OH-). Example: NaCl (Na+ and Cl-). Salts conduct electricity (salts are electrolytes). ACIDS, BASES & SALTS ARE ELECTROLYTES Beware of Tricks . . . Organic Acids have a functional group –COOH, so when you see a compound with carbon and this functional group it is an acid!! Ex: CH3COOH Alcohols have a functional group –OH (hyroxyl), not OH- (hydroxide); alcohols are not bases!! Ex: CH3OH Table K Table L Strong Acids & Bases HCl HBr HI HNO3 H2SO4 HClO3 HClO4 NaOH KOH Ca(OH)2 LiOH RbOH CsOH Ba(OH)2 NH3 is a weak base!! ARRHENIUS THEORY OF ACIDS & BASES An Arrhenius Acid has H and releases H+ in an aqueous solution. The H+ ion is the only positive ion in these solutions. The H+ ions are always attached to H2O forming H3O+ (hydronium ions). An Arrhenius base has OH (hydroxide) and releases OH- (hydroxide ion) in an aqueous solution. The OH- ion is the only negative ion in these solutions. Bronsted-Lowry Theory (Alternate Acid-Base Theory) Bases Accept Acids Donate Bases: H+ acceptor (proton acceptor) Acids: H+ donor (proton donor) Conjugate Acid Base Pairs 1. NH4+ + OH- → NH3 + H2O 2. H3PO4 + NO2- → HNO2 + H2PO4- 3. HI (aq) + H2O (l) → H3O+ (aq) + I- (aq) Some substances can sometimes act like an acid and sometimes act like a base. Examples are H2O & HSO4- Neutralization Reactions ACID + BASE = WATER + SALT (IONIC SOLID) Titration Titration is a lab process in which a volume of a solution of known concentration is used to determine the concentration of another solution. MAVA =MBVB Example 1: What is the molarity of HCl (aq) if 10. milliliters of 4.0 M NaOH (aq) neutralizes exactly 20. milliliters of HCl (aq)? Example 2: What is the molarity of NaOH (aq) if 10. milliliters of 2 M HBr (aq) neutralizes exactly 5. milliliters of NaOH (aq)? pH Scale Movement from one whole number to the next represents a change by a power of 10. Acids: 1-7, [H3O+] > [OH-] Neutral: 7, [H3O+] = [OH-] Bases: 7-14, [H3O+] < [OH-] Meaning of pH & pOH pH = -log[H+] pH = -log[1.0X10-5] = -(-5) = 5 The solution is acidic. pOH = -log[OH-] pOH is the concentration of OH- instead of the concentration of H+ pH + pOH = 14