The Nature of Acid-Base 11/30/2009
advertisement

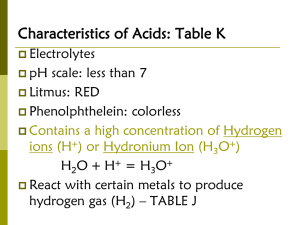
11/30/2009 The Nature of Acid-Base Equilibria Acids and Bases in Aqueous Solution • Arrhenius Definition of Acids and Bases: • An acid is a substance that gives hydrogen ions, H+, when dissolved in water. In fact, H+ reacts with water and produces H3O+. • A base is a substance that gives hydroxide ions, OH-, when dissolved in water. • The neutralization reaction of an acid with a base yields water plus a salt. Chapter 8.1 Some Common Acids • Some common strong acids: • • • • Sulfuric acid, H2SO4 Hydrochloric acid, HCl Nitric acid, HNO3 Perchloric acid HClO4 • Weak acids: • Phosphoric acid, H3PO4 Some Common Bases •Some common strong bases: • Sodium hydroxide, NaOH; • Calcium hydroxide, Ca(OH)2 • Magnesium hydroxide, Mg(OH)2 •Weak base: • Ammonia, NH3 • Acetic acid, CH3CO2H •The Bronsted-Lowry Definition of Acids and Bases: •Acid: Any substance that is able to give a hydrogen ion, H+, to another ion or molecule. H+ ions are also known as a proton. Therefore, acids are those substances that can donate protons. •Base: Any substance that is able to accept a hydrogen ion, H+, from an acid. A base can be neutral or negatively charged, for example, ammonia, NH3 and hydroxide ion, OH-. 1 11/30/2009 •Monoprotic acid : Acids with one proton to donate, such as, hydrochloric acid, HCl; nitric acid, HNO3 •Diprotic acid: Acids with two protons to donate, such as sulfuric acid, H2SO4. •Triprotic acid: Acids with three protons to donate, such as phosphoric acid, H3PO4. •Conjugate acid-base pair: Two substances whose formula differ by only a hydrogen ion, H+. •Conjugate base: The substance formed by loss of H+ from an acid. •Conjugated acid: The substance formed by addition of H+ to a base. Acid Dissociated Constants Water as Both an Acid and a Base •According to Bronsted-Lowry acid-base theory, water is both an acid and a base. Called amphoteric. • NH3 + H2O NH4+ + OH- • CH3COOH + H2O CH3COO- + H3O+ • Strong acids have Ka value much greater than 1. • Weak acids have Ka value much less than 1. • Donation of each successive H+ from a polyprotic acid is more difficult than the one before it, so Ka value become successively smaller. • Most organic acids, which contains –CO2H group, have Ka value near 10-5. •The reaction of weak acid with water can be described by an equilibrium equation. Equilibrium constant, K, and water concentration [H3O+] together makes the acid dissociation constant Ka. Acid dissociation constant is a measure of acid strength. Dissociation of Water • H2O + H2O H3O+ + OH- •Ion product constant for water, Kw: •Kw = [H3O+][OH-] = 1.00 x 10-14 at 25oC. • therefore, [H3O+]=[OH-] = 1.0 x 10-7 M •Product of [H3O+] and [OH-] is a constant. Therefore, in an acidic solution where [H3O+] is large and [OH-] must be small. 2 11/30/2009 Measuring Acidity in Aqueous Solution: pH •A pH value between 0 and 14 is used to indicate concentration of H3O+ or OH- in solution. Mathematically, the pH of a solution is defined as the negative common logarithm of the H3O+ concentration: • pH = -log [H3O+ ] or • [H3O+ ] = 10-pH •Acidic solution: •Neutral solution: • Basic solution: pH < 7 pH = 7 pH > 7 [H3O+ ] > 1.00 x 10-7 M [H3O+] = 1.00 x 10-7M [H3O+ ] < 1.00 x 10-7 M • “Acidity” depends on the concentration of H3O+ ions Acidic: [H3O+] > [OH-] Basic: [H3O+] < [OH-] Neutral: [H3O+] = [OH-] • Notice that neutral does NOT necessarily mean pH 7 • pH is usually quoted with the same number of significant digits as the concentration • The p-scale can also be applied to ionization constants pKa = - log Ka • The larger the value of Ka the smaller the value of pKa and the stronger the acid • From the acid-dissociation constant we can calculate equilibrium concentrations as well as pH 3