Acids and Bases
advertisement

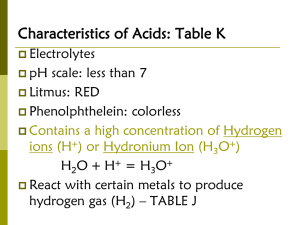
Chapter 19 All aqueous solutions contain hydrogen ions (H+) and hydroxide (OH-) ions. An acidic solution contains more H+ ions than OH-. A basic solution contains more OH- ions than H+ ions. When a solution has the same concentration of H+ and OH- it is said to be neutral. A hydronium ion (H3O+) is a hydrated hydrogen ion. H+ and H3O+ mean the same thing and can be used interchangeably. Arrhenius model • An acid is a substance that contains hydrogen and ionizes in aqueous solution to produce hydrogen ions. • A base is a substance that contains a hydroxide group and dissociates in aqueous solution to produce hydroxide ions. Brønsted-Lowry model • An acid is a hydrogen-ion donor. • A base is a hydrogen-ion acceptor. When a Brønsted-Lowry acid donates a hydrogen ion, a conjugate base is formed. When a Brønsted-Lowry base accepts a hydrogen ion, a conjugate acid is formed. Identify the conjugate acid-base pairs in this reaction. HClO2 (aq) + H2O (l) H3O+ (aq) + ClO2- (aq) 1. Indentify the conjugate acid-base pairs in the following reactions. a. H2SO3 (aq) + H2O (l) HSO3- (aq) + H3O+ (aq) b. HPO42- (aq) + H2O (l) H2PO4- (aq) + OH- (aq) c. HSeO3- (aq) + H2O (l) H3O+ (aq) + SeO32- (aq) An acid that can donate only one hydrogen ion is called a monoprotic acid. For example, hydrochloric acid (HCl) and formic acid (HCOOH). Note: Only those hydrogens that are bonded to electronegative elements are ionizable. Some acids can donate more than one hydrogen ion. For example, sulfuric acid (H2SO4) contains 2 ionizable hydrogen atoms, and is called a diprotic acid. Boric acid (H3BO3) contains 3 ionizable hydrogen atoms, and is called a triprotic acid. An acid with two or more ionizable hydrogens is called a polyprotic acid. The three ionizations of boric acid are as follows. H3BO3 (aq) + H2O (l) H3O+ (aq) + H2BO3- (aq) H2BO3- (aq) + H2O (l) H3O+ (aq) + HBO32(aq) HBO3 2-(aq) + H2O (l) H3O+ (aq) + BO3 3- (aq) 2. Write the steps in the complete ionization of the following polyprotic acids. a. Carbonic acid (H2CO3) b. Chromic acid (H2CrO4) An acid that ionizes completely in dilute aqueous solution is called a strong acid. There are six strong acids that you need to learn, and they are listed on page 603 in your textbook. A weak acid is one that ionizes only partially in dilute aqueous solutions. For a weak acid, a state of equilibrium is reached in which the forward and reverse reactions occur at equal rates. Considering the reaction of the weak acid formic acid HCOOH (aq) + H2O (l) H3O+ (aq) + HCOO- (aq) The equilibrium constant expression of the ionization of formic acid in water is as follows: Ka= [H3O+][HCOO-] [HCOOH] Ka is the acid ionization constant. Ka is the value of the equilibrium constant for the ionization of a weak acid. Ka is a measure of the extent of ionization of the acid. Weak acids have the smallest Ka values. Polyprotic acids have a Ka value for each ionization, and the Ka values decrease for each successive ionization. 3. Write ionization equations and acid ionization constant expressions for the following acids. a. Hydrofluoric acid (HF) b. Hypobromous acid (HBrO) 4. Write the ionization equation and the acid ionization constant expression for the second ionzation of sulfurous acid (H2SO3) in water. Metallic hydroxides are strong bases which dissociate entirely into metal ions and hydroxide ions in aqueous solution. Group 1A and 2A hydroxides are strong bases. A weak base is a base that ionizes only partially in dilute solution to form an equilibrium mixture. Just like there is an acid ionization constant there is a base ionization constant. The Kb value is the value of the equilibrium constant. Kb is smallest for the weakest bases. 5. Write ionization equations and base ionization constant expressions for the following bases. a. Butylamine (C4H9NH2) b. Phosphate ion (PO43-) c. Hydrogen carbonate ion (HCO3-) Pure water self-ionizes slightly to form H3O+ and OH- ions, as shown H2O (l) + H2O (l) H3O+ (aq) + OH- (aq) It can be simplified by removing one water molecule from each side H2O (l) H+ (aq) + OH- (aq) A special equilibrium expression for the self-ionization of water is defined as follows: Kw = [H+][OH-] Kw is called the ion product constant for water. It is the value of the equilibrium constant expression of water. In pure water at 298 K, the concentration of H+ ions and OH- ions both equal 1.0 x 10-7 M, so the value of Kw = 1.0 x 10-14. At 298 K, the OH- ion concentration of an aqueous solution is 1.0 x 10-11 M. Find the H+ ion concentration in the solution and determine whether the solution is acidic, basic or neutral. 6. Given the concentration of either hydrogen ion or hydroxide ion, calculate the concentration of the other ion at 298 K and state whether the solution is acidic, basic, or neutral. a. [OH-] = 1.0 x 10-6 M b. [H+] = 1.0 x 10-7 M c. [H+] = 8.1 x 10-3 M Because the concentrations of H+ ions are often small, the pH scaled was developed. The pH of a solution equals the negative logarithm of the hydrogen ion concentration. pH = -log [H+] The pH scale has values from 0 to 14. Acids have pHs less than 7 and bases have pHs greater than 7. A pH of 0 is the most acidic and a pH of 14 is the most basic. A pH of 7 is neutral. The pOH scale expresses the basicity of a solution. pOH is the negative logarithm of the hydroxide ion concentration. pOH = -log [OH-] If either the pH or pOH are known, the other may be determined by using the following relationship. pH + pOH = 14.00 The pH and pOH values can be determined if eith the [H+] or [OH-] is known. If a certain carbonated soft drink has a hydrogen ion concentration of 7.3 x 10-4 M, what are the pH and pOH of the soft drink? 7. Calculate the pH and pOH of aqueous solutions having the following ion concentrations. a. [H+] = 1.0 x 10-14 M b. [OH-] = 5.6 x 10-8 M c. [H+] = 2.7 x 10-3 M d. [OH-] = 0.061 M When the pH of a solution is known, you can determine the concentrations of H+ and OH-. What are [H+] and [OH-] in an antacid solution with a pH of 9.70? 8. The pH or pOH is given for three solutions. Calculate [H+] and [OH-] in each solution. a. pH = 2.80 b. pH = 13.19 c. pOH = 8.76 Remember, that strong acids and bases dissociate completely in water. This means that for monoprotic acids the concentration is equal to the concentration of the hydrogen ion. In some acids and bases, there are more than one hydrogen ions or hydoxide ions in the compound. 9. Calculate the pH of the following strong acid or strong base solutions. a. 0.015 M HCl b. 0.65 M KOH c. 2.5 x 10-4 M HNO3 d. 4.0 x 10-3 M Ca(OH)2 If you know the pH and the concentration of a solution of a weak acid, you can calculate Ka for the acid. The pH of a 0.200 M solution of acetic acid (CH3COOH) is 2.72. What is the Ka for acetic acid? 10. Calculate Ka for the following acids using the information provided. a. 0.100M solution of sulfurous acid (H2SO3), pH = 1.48 b. 0.200M solution of benzoic acid (C6H5COOH), pH = 2.45 The reaction of an acid and a base in an aqueous solution is called a neutralization reaction. The products of a neutralization reaction are always a salt and water. A salt is an ionic compound composed of a positive ion from a base and a negative ion from an acid. Acid-base neutralizations are used in the procedure called titration, which is a method for determining the concentration of a solution by reacting it with another solution of known concentration. Neutralization reactions proceed until an equivalence point is reached. The equivalence point is the point where the moles of H+ ions and OH- ions are equal. At the equivalence point, a large change in pH occurs that can be detected by a pH meter or an acid-base indicator. An indicator is a dye whose color is affected by pH changes. When a strong acid is titrated with a strong base, the equiv. point is 7. When strong acid, weak base equiv. point is less than 7; when weak acid and strong base the point is greater than 7. In a titration, 53.7 mL of 0.100 M HCl solution is needed to neutralize 80.0 mL of KOH solution. What is the molarity of the KOH solution? 11. A 45.0 ml sample of nitric acid solution is neutralized by 119.4 mL of 0.200 M NaOH solution. What is the molarity of the nitric acid solution? A buffer is a solution that resists changes in pH when moderate amounts of acid or base is added. A buffer is a mixture of a weak acid and its conjugate base, or a weak base and its conjugate acid.