pH in Biology Dissociation of water molecules leads to acidic and
advertisement

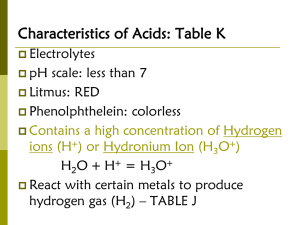
pH in Biology Dissociation of water molecules leads to acidic and basic conditions that affect living organisms Dissociation of Water Sometimes, an H2O molecule loses a H+ ion that is in turn accepted by another H2O Ionization of Water Occasionally, in water, a H+ is transferred between H2O molecules .. H :O : + .. .. H .. .. .. :O:H H:O:H + + H .. .. :O:H - H water molecules hydronium hydroxide ion (+) ion (-) 4 LecturePLUS Timberlake • H+ leaves its electron behind and is transferred as a single proton - a hydrogen ion (H+) • The water molecule that lost a proton is now a hydroxide ion (OH-) • The water molecule with the extra proton is a hydronium ion (H3O+) H2O <=> H+ + OH• This reaction is reversible. • At equilibrium the concentration of water molecules greatly exceeds that of H+ and OH• At equilibrium the concentration of H+ or OH- is 10-7M (25°C) • Adding solutes (acids or bases) changes the concentration of H+ and OHSolutions with more OH- than H+ are basic solutions. Solutions with more H+ than OH- are acidic solutions. Solutions in which concentrations of OH- and H+ are equal are neutral solutions. Pure Water is Neutral Pure water contains small, but equal amounts of ions: H3O+ and OH- H2O + H2O H3O+ + OH- hydronium hydroxide ion H3O+ OH- 1 x 10-7 M ion 1 x 10-7 M 7 LecturePLUS Timberlake Acids & Bases Acids are formed by hydrogen cations Bases are formed by hydroxide anions Acids Increase H+ HCl (g) + H2O (l) H3O+ (aq) + Cl- (aq) More [H3O+] than water > 1 x 10-7M As H3O+ increases, OH- decreases [H3O+] > [OH-] H3O+ 9 OH- LecturePLUS Timberlake Acids • Donate protons (Hydrogen Ions) to water to form hydronium ions • pH 0-6.99 • Taste Sour • Turn litmus paper red • Strong acids completely dissociate to form ions Bases Increase the hydroxide ions (OH-) H2O NaOH (s) Na+(aq) + OH- (aq) More [OH-] than water, [OH-] > 1 x 10-7M When OH- increases, H3O+ decreases [OH] > [H3O+] H3O+ 11 OH- LecturePLUS Timberlake Bases • Donate hydroxide • pH 7.01-14 • Accept protons • Taste bitter • Feel slimy • Turn litmus paper blue • Strong bases completely dissociate to form ions Bases • Base increases [OH- ] • Some bases reduce the H+ concentration directly by accepting hydrogen ions For example, NH3 + H+ <=> NH4+ • Other bases reduce H+ indirectly by dissociating to OH-, which then combines with H+ to form water. For example, NaOH -> Na+ + OHOH- + H+ -> H 2O NH3, A Bronsted-Lowry Base When NH3 reacts with water, most of the reactants remain dissolved as molecules, but a few NH3 reacts with water to form NH4+ and hydroxide ion. NH3 acceptor + + H2 O NH4+(aq) + OH- (aq) donor + 14 LecturePLUS Timberlake Strengths of Acids and Bases Strong acids completely ionize (100%) in aqueous solutions HCl + H2O H3O+ + Cl- (100 % ions) Strong bases completely (100%) dissociate into ions in aqueous solutions. NaOH Na+ (aq) + OH-(aq) (100 % ions) 15 LecturePLUS Timberlake Strong and Weak Acids and Bases Strong acids HCl, HNO3 , H2SO4 Most other acids are weak. Strong bases NaOH, KOH, and Ca(OH)2 Most other bases are weak. 17 LecturePLUS Timberlake Weak Acids/Bases –the binding and release of hydrogen ions are reversible. –At equilibrium, there will be a fixed ratio of products to reactants –Eg: NH3, carbonic acid –Carbonic acid (H2CO3) is a weak acid: • H2CO3 <=> HCO3- + H+ Measuring Acidity and Alkalinity • In any solution [H+] [OH-] = 10-14 – In a neutral solution, [H+] = 10-7 M and [OH-] = 10-7 M • Adding acid to a solution shifts the balance between H+ and OH- toward H+ and leads to a decline in OH-. – If [H+] = 10-5 M, then [OH-] = 10-9 M