Name: ____________________________________________ Date: ____________________ Block: ____________ 1. Anion –
advertisement

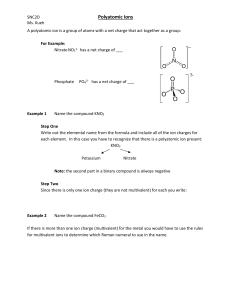
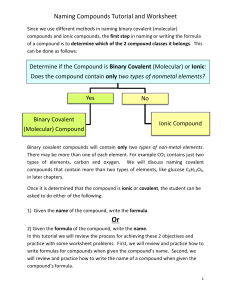
Name: ____________________________________________ Date: ____________________ Block: ____________ Chapter 6 Vocabulary / Questions 1. Anion – 2. Cation – 3. Ion – 4. Binary compound – 5. Chemical formula – 6. Formula unit – 7. Ionic compound – 8. Law of multiple proportions – 9. Law of definite proportions – 10. Molecular compound – 11. Molecular formula – 12. Molecule – 13. Monatomic ion – 14. Polyatomic ion – 15. Ternary compound – 16. Explain what a polyatomic ion is: 17. Write the formulas for the following compounds: a. Potassium permanganate ____________________________________________ b. Dichlorine heptaoxide ____________________________________________ c. Trisilicon tetranitride ____________________________________________ d. Calcium nitrate ____________________________________________ e. Calcium nitrite ____________________________________________ f. Calcium nitride ____________________________________________ g. Iron(II)oxide ____________________________________________ h. Cobalt(I)phosphide ____________________________________________ i. Chromium(VI)fluoride ____________________________________________ 18. State the number of electrons either lost or gained in the following: a. Br_________________ d. Fe+3 _________________ + +2 b. Na _________________ e. Cu _________________ c. As-3 _________________ f. N-3 _________________ Chapter 8 Vocabulary / Questions 19. Balanced equation – 20. Catalyst – 21. Chemical equation – 22. Coefficient – 23. Combination (synthesis) reaction – 24. Combustion reaction – 25. Decomposition reaction – 26. Double displacement (replacement) reaction – 27. Single displacement (replacement) reaction – 28. Spectator ion – 29. What is a characteristic of every combination reaction? 30. What is a distinguishing feature of every decomposition reaction? 31. What substance is common to all combustion reactions? ____________________________________ 32. Balance each of the following reaction AND IDENTIFY its type: a. ___Hf + ___ N2 ___Hf3N4 Type: _______________________ b. ___ Mg + ___H2SO4 ___MgSO4 + ___ H2 Type: _______________________ c. ___C2H6 + ___ O2 ___CO2 + ___H2O Type: _______________________ d. ___Pb(NO3)2 + ___ NaI ___PbI2 + ___NaNO3 Type: _______________________ e. ___Fe + ___O2 ___Fe3O4 Type: _______________________ f. ___Pb(NO3)2 ___PbO + ___NO2 + ___ O2 Type: _______________________ g. ___Hg(NO3)2 + ___NH4SCN ___Hg(SCN)2 + ___NH4NO3 Type: _____________________ 33. What does the presence of an –ide ending on the name of an ion tell you about that ion? 34. What kind of ions will metals typically make? __________________________ 35. What kind of ions will nonmetals typically make? __________________________