IPC Notes
advertisement

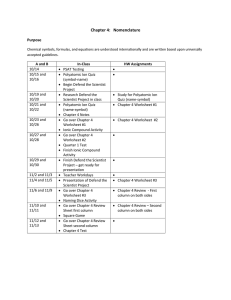
IPC Notes Polyatomic Ion Names & Formulas Polyatomic Ions A polyatomic ion is a charged covalently bonded group of atoms. ex) sulfate is SO4-2 Common Polyatomic Ions Nitrate = NO3-1 Sulfate = SO4-2 Phosphate = PO4-3 Ammonium = NH4+1 Carbonate = CO3-2 Hydroxide = OH-1 Common Polyatomic Ions Nitrate = NO3-1 Sulfate = SO4-2 Phosphate = PO4-3 Ammonium = NH4+1 Carbonate = CO3-2 Hydroxide = OH-1 NOTE: There are many more polyatomic ions that this short list. You will use about 30 of them in Chemistry. Writing Formulas 1) Write the symbol of the first ion with its oxidation number. 2) Write the symbol of the second ion with its oxidation number. Writing Formulas 3) Criss cross the numbers, not the + or – sign. 4) If a subscript needs to be used with a polyatomic ion, write parenthesis around the polyatomic ion first and then put the subscript at the bottom right. Writing Formulas Write the formula for: aluminum sulfate Writing Formulas Write the formula for: potassium nitrate Writing Formulas Write the formula for: calcium phosphate Writing Formulas Write the formula for: magnesium hydroxide Writing Formulas Write the formula for: calcium carbonate Writing Formulas Write the formula for: ammonium phosphate Writing Names 1) Write the name of the first ion. 2) Write the name of the second ion. If the second ion is a polyatomic ion, leave the name alone. If the second ion is just an element, change the ending to –ide. Naming Compounds Write the name for: MgSO4 Naming Compounds Write the name for: KNO3 Naming Compounds Write the name for: Al2(CO3)3 Naming Compounds Write the name for: NH4F Naming Compounds Write the name for: NH4NO3 Naming Compounds Write the name for: Ca(OH)2