OL fall semester exam review pap 2014 KEY
advertisement

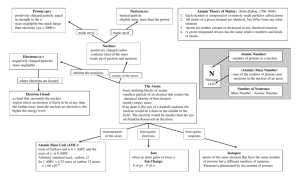
PAP FALL SEMESTER EXAM REVIEW 2014 KEY Explain the difference between each pair of terms. 1. Pure substances vs. mixtures a. Pure substance – substance that has a fixed (non-changing) composition with the same properties and characteristics in every sample (elements and compounds) b. Mixtures – substance that has a variable (changing) composition made up of two or more types of matter 2. elements vs. compounds a. element – pure substance made up of only one type of atom (anything on the periodic table) b. compounds – substance that is made from the chemical combination of two or more different types of atoms 3. physical properties vs. chemical properties a. physical – a characteristic that can be observed or measured without changing the identity of the substance b. chemical – the ability of a substance to undergo a change that transforms it into a different substance 4. extensive vs. intensive properties a. Intensive Property – properties based on the IDENTITY of a substance b. Extensive Property - properties based on the AMOUNT of a substance 5. accuracy vs. precision a. accuracy – being close to the correct answer or accepted value b. precision – all measurements or values being close together 6. Identify the described properties as Physical (P) or Chemical (C) and Intensive (I) or extensive (E). a. b. c. d. odorless P I mass of 5.00 grams P E tarnishes rapidly in air C I boiling point of 883 C P I e. soft, silver-white P I f. reacts violently with water C I g. reacts with acid C I 7. Classify changes of matter as physical or chemical. ____P____ A piece of metal is heated until it turns red. ____C____ Aluminum and oxygen react to produce aluminum oxide. ____C____ An iron nail rusts. _____P___ A piece of copper metal is hammered into a thin sheet. _____P___ An ice cube melts. _____C___ ____P____ Hydrochloric acid neutralizes sodium hydroxide to form sodium chloride & water. Magnesium chloride is dissolved in water 8. Define the following terms. atomic number – number of protons in an atom (also number of electrons in neutral atom) mass number – sum of protons and neutrons in the nucleus of an atom average atomic mass – average mass of all naturally occurring isotopes of an atom (on the periodic table) isotope – atoms of the same element with different mass numbers (same protons, different neutrons) ion – an atom with a charge (uneven amount of protons and electrons) valence electrons – electrons in the outer shell of the atom (used in chemical bonding) Octet Rule – atoms gain, lose and share electrons to get 8 valence electrons (full outer shell) electronegativity – the ability of an atoms to pull electrons towards itself in a bond ionization energy – energy required to remove a valence electron atomic radius – the size of an atom ionic radius – the size of an ion 9. Describe each of the states of matter in terms of shape, volume, and compressibility. Include a picture of the state of matter at the molecular level. 10. What must happen in order for a change in state of matter to occur? Add or remove energy 11. Arrange each group of elements in order of increasing ionization energy. a. F, Br, I, Cl I Br Cl F b. Ga, Al, Tl, B Tl Ga Al B c. Al, Si, Cl, S Al Si S Cl 12. For each group of elements, choose the element with the smallest atomic radius. a. Na, Li, K, Fr Li b. Tc, Rh, Zr, Y Rh 13. For each group of elements, choose the element with the greatest electronegativity. c. Hf, Cs, Pb, Pt Pb a. He, Rn, Xe, Ar b. As, N, P, Bi He N c. Ba, Hf, Os, Hg Hg 14. In general, ionization energies of the elements increase across each period and generally decrease down each group. a. Why? i. ionization energy decreases down a group because The farther away from the nucleus an electron is, the easier it is to remove because the Coulombic attraction of the protons has less hold on the negative charge of the electron. ii. Ionization energy increases across a period because the increased number of protons in the nucleus, causes an increase in the force of attraction 15. In general, electronegativities of the elements increase across each period and generally decrease down each group. a. Why? i. electronegativity decreases down a group because because The farther away from the nucleus an electron is, the harder it is to pull because the Coulombic attraction of the protons has less hold on the negative charge of the electron. ii. electronegativity increases across a period because the increased number of protons in the nucleus, causes an increase in the force of attraction 16. In general, the atomic radii of the element increase down a group and decrease across each period. a. Why? i. Period trend - decreases across a period because of the increase in positive charge of nucleus (more p+ added) creates a greater Coulombic attraction on the e– towards nucleus while the new e– are added within same energy level ii. Group Trend - increases down a group because e– are added to higher energy levels, farther from nucleus, making radius larger 17. The modern periodic table is arranged according to increasing atomic number. 18. Label the number of valence electrons for each group: 1 __1__ 2___2___ 13__3____ 14 __4___ 15 __5___ 16 ____6__ 17 ___7____ 18 ___8___ 19. Elements in the same family have similar properties. 20. Describe the location of each of the following groups of elements and identify some common properties and uses of elements in the family. metalloids Stair step between metals and non-metals Properties in between metals and non-metals o Si: brittle but semi-conductor Many metalloids are used in computer chips to conduct electricity without conducting too much heat halogens "halogen" means "salt-former" and compounds containing halogens are called "salts" Most reactive non-metals exist in all three states of matter at room temperature alkaline earth metals They are harder, denser, and stronger than the alkali metals with higher melting points They are so reactive they are not found as free elements in nature Noble gases Nonreactive due to their complete valence shells Do not easily gain or lose valence electrons All gases at room temperature used in lighting and balloons transition metals Metals with typical metallic properties and uses Less reactive than group 1 and 2 metals – some are so unreactive that they exist in nature as free-elements Used in jewelry, coins, building materials alkali metals Most reactive metals that do not occur freely in nature Slivery-white and softer than most other metals (to the point that they can be cut easily with a knife) can explode if they are exposed to water 21. Contrast the properties of metals and nonmetals. Explain the location of each on the periodic table. Metals - Properties: Nonmetals - Properties: – lustrous (shiny) – Dull appearance – good conductors of heat & electricity – Brittle when solids – malleable & ductile – Do not conduct heat or electricity well – solids at RT (except Hg) – May be solid, liquid or gas at RT 22. Identify the location in the atom, mass, charge, and volume for each of the subatomic particles a. proton nucleus, 1 amu, +1, no volume b. neutron nucleus, 1 amu, no charge, no volume c. electron electron cloud, 0 amu, -1, all volume of the atom 23. Describe the experiments and their conclusions for: a. JJ Thompson - used the cathode ray tube to discover the 1st subatomic particle - the electron b. Ernest Rutherford - gold foil experiment - expected all of the radiation to pass through and was very surprised when some of the particles were deflected - led to the discovery of the NUCLEUS and the theory of the nuclear atom. Also discovered the proton. 24. List the five points of Dalton’s Atomic Theory and indicate which ones are known to be incorrect today and WHY they are incorrect. All matter is composed of tiny particles called atoms. TRUE Atoms of an element are identical in size, mass and other properties. FALSE (isotopes) Atoms cannot be created, destroyed or subdivided. FALSE (subatomic particles) Atoms of different elements can combine in simple whole number ratios to form compounds. TRUE In chemical reactions, atoms are combined, separated or rearranged. TRUE 25. Sketch the evolution of the atomic model. Include the name of the model and the scientist associated with it. 26. An element consists of three naturally occurring isotopes with the following mass numbers: 24, 25 and 26. The relative abundances of these three isotopes are 78.70, 10.13 and 11.17 percent respectively. Calculate the average atomic mass and identify the element. Show your work! (24.32 amu) (24 x .7870) + (25 x .1013) + (26 x .1117) 27. A naturally occurring element, X, exists as 92.21% 28X, which has an atomic mass of 27.97693 amu, 4.70% 29X, which has an atomic mass of 28.97659 amu, and 3.09% 30X, which has an atomic mass of 29.97376amu. Calculate the average atomic mass and identify the element. Show your work! (28.11 amu) (28 x .9221) + (29 x .0470) + (30 x .0309) 28. Complete the following chart. 29. The electron configuration s2p6 represents a. A halogen b. A metalloid c. A noble gas Write the complete electron configuration, Noble gas configuration, orbital notation, dot diagram, and quantum numbers for the following elements. 30. Nitrogen (N)– number of electrons 7 complete e – configuration: Dot diagram: 1s22s22p3 31. Magnesium (Mg) – number of electrons 12 complete e – configuration: 2 2 6 Dot diagram: 2 1s 2s 2p 3s 32. Define the following terms chemical bond – any force that holds two or more atoms together cation – positively charged ion (metals, lost electrons) anion – negatively charged ion (nonmetals, gained electrons) ionic bond – electrostatic attraction between cations and anions after a transfer of electrons covalent bond – two nonmetals sharing electrons metallic bond – pure metals bonding through the sea of electrons electron sea model – stationary metal nuclei release their valence electrons which are free to float between them all polarity – the uneven distribution of charge across a molecule 33. Identify if the following are ionic or covalent, then write names for the following compounds Ionic - Sodium sulfate Na2(SO4) Ionic - Tin (II) hydroxide Sn(OH)2 Ionic - Ammonium sulfide (NH4)2S Ionic - Iron (II) carbonate Fe(CO3) Ionic - Barium phosphide Ba3P2 Covalent - Carbon dioxide CO2 Ionic - Lead (IV) hydroxide Pb(OH)4 34. Identify if the following are ionic or covalent, then write formulas for the following compounds Ionic - Sr(NO3)2 strontium nitrate Ionic - Cd(NO3)2 cadmium nitrate Covalent - P4Cl10 tetraphosphorus decachloride Ionic - Li2(CO3) lithium carbonate Covalent - N3O7 trinitrogen heptoxide Ionic - Cu(C2H3O2)2 copper (II) acetate 35. Briefly describe what happens that allows you to see colors in the flame tests and the gas tubes. When energy is added to an atom, an electron jumps to a higher energy level (excited state). It does not stay in the excited state for long, so as it falls back to its original location (ground state), it releases energy in the form of electromagnetic radiation which based on energy, frequency and wavelength will show as a different color. 36. Describe the relationships between energy, wavelength, and frequency. a. Wavelength and Frequency - Inversely related b. Wavelength and Energy - Inversely related c. Frequency and Energy - Directly related d. Speed of different types of electromagnetic radiation - Constant 37. What is the energy of a quantum of light with a frequency of 4.31 X 1014 1/s or Hz? (2.86 x 10-19 J) E = hv = (6.63 x 10-34 Js)( 4.31 X 1014 Hz) = 2.86 x 10-19 J 38. A certain violet light has a wavelength of 413 nm. What is the frequency of the light? (7.26 x 1014 Hz) c = vλ v = c/λ = (3.0 x 108 m/s) / (413 x 10-9 m) = 7.26 x 1014 Hz 39. What is the energy of light with a wavelength of 662 nm? (3.00 x 10-19 J) c = vλ v = c/λ = (3.0 x 108 m/s) / (662 x 10-9 m) = 4.53 x 1014 Hz E = hv = (6.63 x 10-34 Js)( 4.53 X 1014 Hz) = 3.00 x 10-19 J 43. Determine the Lewis structure, VSEPR shape, bond angle, hybridization, and polarity of the following: a. CO, e. PH3 f. b. CO2 c. CCl4 d. H2O HCN