CHM 412 Alkanes and Alkenes 1-10-13
advertisement

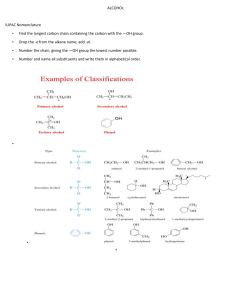
Content: Alkane: Structure and physical properties (boiling point and solubility), Nomenclature (naming) Preparation (hydrogenation of alkenes, hydrolysis of Grignard reagents) Alkane reactions (halogenation/free radical substitution, combustion) Sources and uses Bpt vs C-chain length As Chain length^, # e-^, therefore LDF's^, so bpt^ Source: http://www.chemistryrules.me.uk/junior/organic.htm Very good for introductory organic Chem! As branching ↑, Surface area ↓, hence VdW’s ↓, bpt ↓ ^^ Reference http://chemed.chem.wisc.edu/chempaths/GenChem-Textbook/Properties-of-Alkanes-917.html Nomenclature for alkanes • Find the longest carbon sequence (either a chain or within a ring). That forms the ROOT / PARENT / PRIMARY name. • Name ‘branches’ as prefixes in alphabetical order (note: do not use di, tri, tetra etc in the alphabetical ordering) • Use numbering so numbers are as low as possible. • Ring structure have the prefix ‘cyclo’ rest of name. • Letters are separated from numbers using a hyphen • Numbers are separated from each other using a comma • ‘special groups’ (commonly occur, so given their own name and they remain in widespread use) https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/nomen1.htm Very good page for nomenclature Hydrocarbon preparation • Hydrogenation of alkenes. H H + C C H H H metal catalyst: Pt or Rh or Ni H H H H H H H • Hydrolysis of Grignard reagents. Grignard reagent (organo magnesium) Bt + + Mg in dry ether BrMg - + H O H H ( + MgOHBr) Note: This example shows a trivial example You would NEVER do this to get a simple, readily available alkene You would only do it to make a rare or complicated alkane. New bond + • Or by other Grignard reactions: OTs + BrMg Grignard (organo magnesium) Alkane reactions (Functionalisation of alkanes) http://driesnuyts.blogspot.com/2012/04/alkanes-and-radicals-introduction.html Combustion of alkanes • Fuels (e.g. the RM1.92 RM2.10 Ron95) are made up mostly of alkanes. In excess oxygen • CnH2n+2 + (1.5n+½)O2 nCO2 + (n+1)H2O • The process is exothermic and causes a large increase in volume and entropy. Application: Sources and uses • Uses: as fuels (e.g. Kerosine), solvents (e.g. hexane), chemical feedstock • Source: Primarily from the Crude oil which is a complicated mixture of many compounds. Some long hydrocarbons are broken up to produce smaller, often more useful hydrocarbons. Conformations • Alkane chains and rings are in a constant state of motion/vibrations (due to background thermal energy). Alkane vhains can rotate about the C-C and rings can flex. Each ‘snapshot’ of the orientation of the molecule has it’s own energy. Alkane ‘chain’ conformers 1 cal = 4.18 Joules http://chemistry.umeche.maine.edu/CHY251/Butane.html Alkane ring conformers Energy profile of cyclohexane conformers… http://www.stolaf.edu/depts/chemistry/courses/toolkits/247/js/cyclohex/ Nomenclature practice: Alkenes H H H H H H Ethene H H C C H H H H Butene? H C C H H H H Propene H C C C H H H H C C C C C C H H But-1-ene H trans-But-2-ene 3-Methyl-cyclohexene Bpt of alkenes v.similar to alkanes. Why? Similar # e- (2 less than alkane) Dehydration of alcohols Use a dehydrating agent e.g. c.H2SO4 or c.H3PO4 or Al2O3 and heat. Different temps used can give different products!!! http://www.transtutors.com/chemistry-homework-help/alcohols/dehydration-of-alcohols.aspx Dehydration of alcohols Mechanism (how reaction happens) http://www.education.com/study-help/article/nomenclature-structure/ Prep of alkenes Dehydrohalogenation http://www.personal.psu.edu/users/t/h/the1/e2.htm Dehydration of alcohols Zaitsev's (Saytzeff) rule: For Dehydration and dehydrohalogenation, the more substituted alkene product is major product http://www.mhhe.com/physsci/chemistry/carey/student/olc/graphics/carey04oc/ref/ch05eliminationreactions.html Markovnikov Rule: Cation (usually H+) adds into the compound to make the most substituted carbocation. Familiarity with carbocation stability is crucial in organic chemistry https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/addene1.htm Addition reactions of alkenes (X-Y) http://www.chemguide.co.uk/mechanisms/eladd/whatis.html#top http://www.chemguide.co.uk/mechanisms/eladd/whatis.html#top Overall: Can also occur via free radical mechanism http://mhschemf6.blogspot.com/2011/05/alkene.html Markovnikov Rule: Cation (usually H+) adds into the compound to make the most substituted carbocation. Addition reactions of alkenes (X-X) http://www.masterorganicchemistry.com/reaction-guide/bromination-of-alkenes-with-br2-to-give-dibromides/ Aleken + AQ Br2 • Gives a halohydrin (+ monobormo product) http://en.wikibooks.org/wiki/Organic_Chemistry/Introduction_to_reactions/Halohydrins Alkenes with conc. H2SO4 • See the similarity with the other conc. acids https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/addene1.htm C=C with KMnO4 (Baeyer test) COL/RT condition: Purple colour of permanganate solution decolourises on addition to colourless alkene. Brown solids (MnO2) procuded in neutral or alkaline conditions. Very pale pink (will look almost colourless, in acidic solution) C=C with KMnO4 (Baeyer test) HOT CONCENTRATED and ACIDIFIED KMnO4 http://www.chemguide.co.uk/organicprops/alkenes/kmno4.html Ozonolysis (cleavage using ozone, O3) http://en.wikipedia.org/wiki/Ozonolysis Ozonolysis (cleavage using ozone, O3) Overall: http://en.wikipedia.org/wiki/Ozonolysis As with all info on these slides, you should try and condense it into simple reaction equations (as above) to help begin the process of ‘connecting’ reactions. The overall equation is just to summarise the reaction and remind you. Alkene (partial summary of) reactions. C=C - Applications and use • Polymers (addition polymers – poly(styrene), PVC, poly(propene) • Raw materisals for making R-X, R-OH and carbonyls 2.3 Aromatic compounds • Answers to practice (L to R, top to bottom) Butane 2-Methylbutane 3,3-Diethyl-4-methylhexane Propylcyclopentane (3-Methyl-2-cyclopentylheptane 3,4-Diisopropyl-2,5,6-trimethylheptane Aromatic compounds (Benzene) http://en.wikibooks.org/wiki/A-level_Chemistry/OCR_%28Salters%29/Reactions_of_arenes 3.0 Aliphatic alcohols Nucleophilic substitution in alcohols (1) H R C : O + H H H (2) .. ( _ ) Nu _ R C H (1) New bond forms between C and Nu (sharing Nu's e- pair) (2) Old bond breaks between C and O Nu + :OH