Topic: Functional Group #5
advertisement

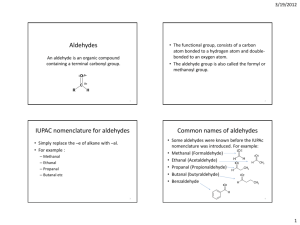
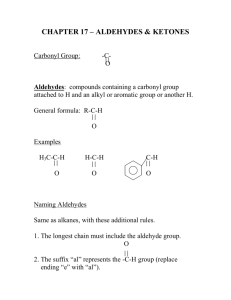
Topic: Functional Group #5-Aldehydes Do Now: Write the molecular formula for the two sugars on the right. Are they isomers? O • General formula: RCH or RCHO • Carbonyl group just like ketone except always at end of aldehyde – Attached to Carbon chain on one side and a H on the other Properties = same as ketones O R C=O + H - R C=O + H - H Aldehydes are polar! Dissolve in water Dipole-Dipole interactions Boiling point: higher than alkanes (same # C’s) + - H Acetaldehyde (ethanal) = important aldehyde • Occurs naturally in • Coffee • Bread • Ripe fruit • Produced by plants • Produced by the partial oxidation of ethanol in the liver and may be a contributing factor to hangovers from alcohol consumption • It’s an air pollutant resulting from combustion (car exhaust and cigarette smoke) Flavoring agents Naming • Find name of alkane with same # of C’s – change the -e to -al • Never need #’s for aldehydes – b/c functional group always on end C O HCH Methanal common name = formaldehyde O H HCCH H Ethanal common name = acetaldehyde CH3CH2CH2CHO CHO ending indicates aldehyde 4 Carbons so base name is butane Drop -e and add al butanal