Unit 1 Self-Quiz, pages 118–119
advertisement

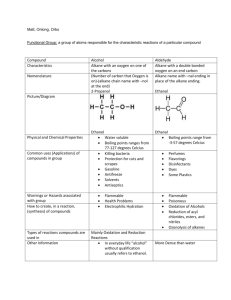
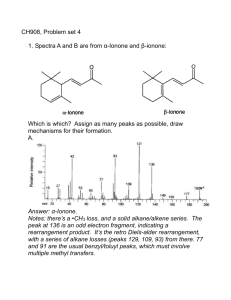
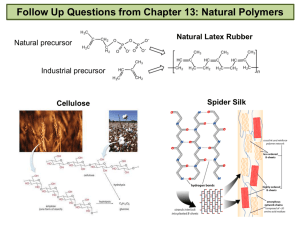
Unit 1 Self-Quiz, pages 118–119 1. (c) 2. (d) 3. (c) 4. (b) 5. (c) 6. (d) 7. (d) 8. (c) 9. (c) 10. (c) 11. (b) 12. (d) 13. (b) 14. (c) 15. (a) 16. (d) 17. (a) 18. (b) 19. (c) 20. (d) 21. (b) 22. (b) 23. False. Methane is the simplest alkane. 24. True 25. True 26. False. The general chemical formula for an alkane is CnH2n+2. 27. True 28. False. The formula of benzene is C6H6. 29. False. The structure of aromatic hydrocarbons is based on a 6-carbon ring with six identical carbon-carbon bonds. 30. False. A tertiary alcohol is an alcohol in which the hydroxyl group is bonded to a carbon with three alkyl groups bonded to it. 31. False. The compound 2-butanone is a ketone. 32. False. An ether contains an oxygen atom between two alkyl groups. 33. False. Aldehydes and ketones are polar. 34. True 35. True 36. True 37. False. Oils have a lower melting point than fats. 38. False. Soap is the sodium salt of a fatty acid. 39. False. Diethylamine is a secondary amine. 40. False. Polymers are large molecules that are built from smaller units called monomers. 41. True 42. True Copyright © 2012 Nelson Education Ltd. Unit 1: Organic Chemistry U1-1 43. False. Nylon is formed in a condensation polymerization reaction. 44. False. Nylon was invented to replace silk. 45. True 46. True Copyright © 2012 Nelson Education Ltd. Unit 1: Organic Chemistry U1-2