Simple Distillation Lab: Separating Colored Water
advertisement

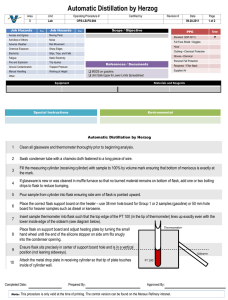
Simple Distillation Distillation is the process of vaporizing a liquid, condensing the vapor, and collecting the condensate in another container. It is one of the principle methods of purifying a liquid mixture. Distillation is a physical separation since no new substance is created at the end of the process. Rather, two pure substances are physically separated from each other. Distillation Theory Heat is added to the liquid mixture, causing the volatile substance to vaporize. In the diagram below, a thermometer that is in contact with the vapor will record the temperature at which vaporization occurs. The temperature will remain constant once the boiling point of the substances has been reached. The vapor then passes through the condenser, which re-liquefies the vapor and passes it into the receiving beaker. Salt Water Distillation (Desalinization) Since sodium chloride (NaCl, or table salt) does not vaporize easily, it can be separated from water using the simple distillation set up shown below. We will use boiling chips which will help spread out the bubbles so that the flask does not "bump." Distillation of Water Containing Food Coloring This activity will introduce you to distillation. Make TWO data tables in which you will record: initial volume of water to the correct number of significant figures! volume of distillate collected to the correct number of signification figures! volume of solution NOT distilled Also, you will record the temperature every 2 minutes during the distillation! This is your second data table. Procedure: 1. Obtain approximately 20 mL of colored water. 2. Place the water in the Erlenmeyer flask, add a few boiling chips and make sure the stopper is on securely! 3. Light the Meker burner and place it under the flask. 4. MAKE OBSERVATIONS OF THE FLASK - note the temperature when the vapor entered the condenser. Also note the color of the solution in the flask and the color of the solution in the beaker. 5. Record the time and temperature as stated above. 6. When the flask is ALL MOST DRY, remove the flame. DO NOT BOIL THE FLASK DRY! 7. Using a graduated cylinder, measure the amount of distillate you collected. 8. Determine the percent of pure water distilled using the following equation: % distilled water = volume of distillate (mL)/volume of starting solution X 100 9. Draw a graph of the temperature vs. the time (which one is the independent variable and which is dependent? The independent variable will be displayed on the "x" axis). Pre-lab questions - You cannot begin the lab until this is completed! 1. How will the contents in the beaker be different that the contents in the Erlenmeyer flask? 2. Will you be measuring the temperature of the liquid in the flask or the vapor? Explain your answer. 3. Differences in what physical property will be used to separate the coloring from the water?