Lab Quiz-Foul Water
advertisement
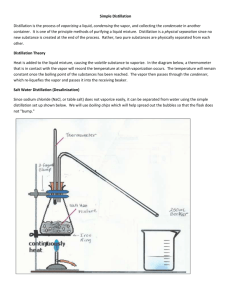
Name_______________________ Period_____ Lab Quiz-Foul Water (18 points) 1. If you recovered 10.3 mL of “pure” water from an original foul water sample that contained 83.2 mL, what percent of the original foul water sample did you recover. Show your work!!! Use correct significant figures!!!! (3 points) 2. What did I discard as the first portion of the recovered liquid during the distillation? (1 point) 3. Name two liquids from the distillation demonstration that DID conduct electricity? (2 points) - 4. What is the difference between a molecular compound and an ionic compound? (1 point) 5. Explain one method of filtration and what it removed from the water. BE SPECIFIC!!!! (2 points) 6. What is the difference between distilled water and deionized water? (1 point) 7. What is the purpose of the Oil-Water separation? Be Specific? (2 points) 8. What processes describe the path of the substance beginning in the glass bulb and ending in the beaker. (3 points) 9. Why was some liquid left in the distilling flask at the end of the demonstration? (1 point) 10. Draw an Erlenmeyer flask and describe what we used it for in this lab. (2 points)