Distillation Demo Worksheet: Chemistry Separation
advertisement

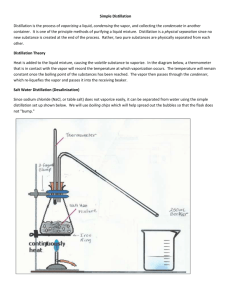
DISTILLATION DEMO Name: Period ________ Date_______________ A liquid sample of unknown composition arrives in your laboratory. Although the sample appears uniform throughout, you have been told that it is a mixture of at least two different liquids. You set up the distillation apparatus pictured below to separate the sample. Questions: 1. What type of mixture are you separating? _____ 2. What role does the burner have in the distillation process? _____ ________________________________________________________________________________ Could the solution be distilled without the burner? Why? _____ ________ 3. You notice that your sample begins to boil at 96.2°C and liquid begins to drip into the collection flask. What is happening? _____ ________________________________________________________________________________ ________________________________________________________________________________ 4. Why is it a good idea to remove the collection flask and replace it with another as soon as the temperature of the solution begins to rise again? _____________________ _________________________________________________________________________________ 5. The temperature rises steadily and the solution begins to boil again at 102.3°C. What is happening now? ________________________ 6. The temperature continues to rise until it reaches 108°C and the rest of the solution boils. How many different liquids were contained in your sample? Explain. _______________ 7. As you complete the distillation, you notice a white, crystalline film lining the inside of the flask that contained the original sample. What could this substance be? _________ Why didn’t you notice it in the original solution? _______________ _________________________________________________________________________________