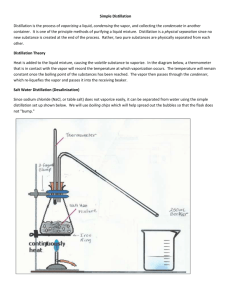
Lab Distillation: Predicting Unknowns in a Mixture Safety: Safety glasses must be worn due to glassware use and heating. Be careful around hot water and the hot plate. The unknown substances will not hurt your skin. Purpose: In this lab, you will get an introduction to distillation. Procedure 1. The lab assembly should resemble the following. Be careful setting it up. We will use an Erlenmeyer flask instead of the Florence flask showing the diagram below. 2. You will need two distillate beakers. Be careful not to twist and turn the glassware. It will break. a. ‘Water in’ collects to the faucet b. ‘Water out’ drains into the sink 4. Measure out a 50 ml sample of the mixture to be distilled and pour it into the flask. 5. Put a few boiling chips to put into the flask. 6. CAREFULLY reconnect the glass tubing to the condenser. 7. Get approval on your setup from your teacher before moving onto the next step. 8. Turn on the water from the sink. This will start the condenser. 9. Turn on the hot plate to a setting of 5 (halfway). This will slowly heat the mixture. 10. When the thermometer reaches 40oC, start recording the temperature every 30 seconds. Make a note at which temperature the liquid starts to collect in the distillate beaker. Temperature should be relatively constant at this moment. 11. If the temperature begins to rise again replace the beaker with the second beaker. 12. Continue heating and recording the temperature every 30 seconds until the second substance just begins to distill. Record the temperature at which the first drop enters the distillate 2 beaker. CAUTION: DO NOT ALLOW ALL OF THE LIQUID TO BOIL FROM THE FLASK!!! 13. Turn off the heat and allow the equipment to cool. 14. Pour the rest of the mixture into the sink without putting the boiling stones into the drain. Table – Time versus Temperature Distillation Data Time (Minutes) Temperature (oC)