Study Guide Ch 6 Chemical Bonding Name:___________________Block#______
advertisement
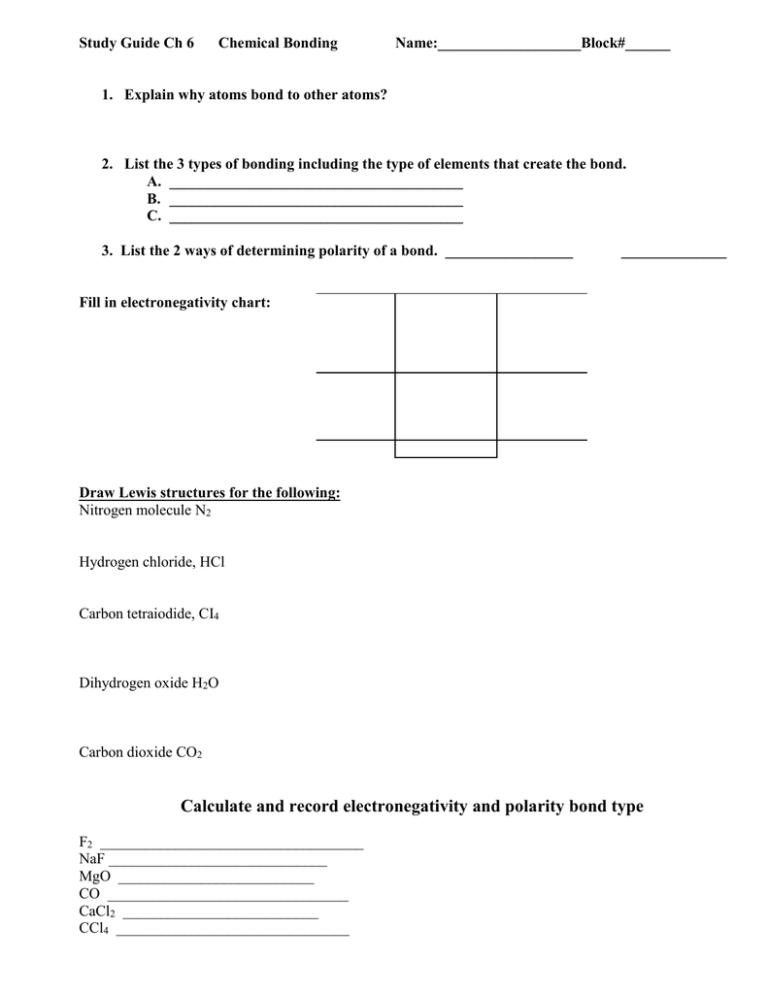
Study Guide Ch 6 Chemical Bonding Name:___________________Block#______ 1. Explain why atoms bond to other atoms? 2. List the 3 types of bonding including the type of elements that create the bond. A. _______________________________________ B. _______________________________________ C. _______________________________________ 3. List the 2 ways of determining polarity of a bond. _________________ ______________ Fill in electronegativity chart: Draw Lewis structures for the following: Nitrogen molecule N2 Hydrogen chloride, HCl Carbon tetraiodide, CI4 Dihydrogen oxide H2O Carbon dioxide CO2 Calculate and record electronegativity and polarity bond type F2 ___________________________________ NaF _____________________________ MgO __________________________ CO ________________________________ CaCl2 __________________________ CCl4 _______________________________ Compare and Contrast Ionic Bond _______________________ _______________________ _______________________ _______________________ _______________________ _______________________ (vs) Covalent bond _________________________ _________________________ _________________________ _________________________ _________________________ _________________________ 7. Explain the intermolecular force that contributes to the high boiling point of water. __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ __________________ 8. Why does water expand when frozen? __________________________________________________________________________________ __________________________________________________________________________________ __________________________________________________________________________________ 9. Compound A has a higher melting point and boiling point than compound B. At the same temperature compound B vaporizes faster than compound A. Which of these compounds would you expect to be ionic? Why? __________________________________________________________________________________ __________________________________________________________________________________ Draw Lewis Structure and predict the VSPER Geometric Shape H2O IBr CH3Br NH3 O2 O3 AlP3 Explain Octet Rule: _____________________________________________________________________________________ _____________________________________________________________________________________ _____________________________________________________________________________________