Chemistry Benchmark II Study Guide
advertisement

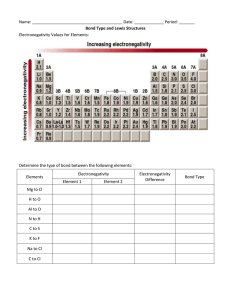
Chemistry Benchmark II Study Guide 1. Write the formula for the following: a. Iron (II) oxide d. ammonium phosphate gf. nitrogen dioxide b. calcium phosphate e. copper (II) bromide h. titanium (IV) oxide c. potassium bromide f. magnesium hydroxide i. xenon hexafluoride 2. What is a binary compound? What is a ternary compound? 3. Write formulas for a. hydroiodic acid e. hyrdrosulfuric acid i. hypochlorous acid 4. Name these: a. NaCl g. HgO m. BF3 b. carbonic acid f. hydrochloric acid j. perchloric acid b. KI h. Fe2O3 n. NO c. CsBr i. MnO2 o. N2O5 c. phosphorous acid g. chloric acid d. Rb2O j. PbCl4 p. CCl4 5. Find the oxidation number assigned to: a. S in H2SO4 b. P in PCl5 c. S in SO3 6. Name these. a. NO2 c. SO3 b. N2O d. sulfuric acid h. chlorous acid e. SrI2 k. CuI q. P4O6 7. How many valence electrons do elements have in the following groups? a. alkali metals b. alkaline earth metals c. halogens f. CuCl l. SnBr4 r. N2O3 d. noble gases 8. Write the complete electron configuration, noble gas configuration, Lewis dot structure, and orbital notation for: a. Sr b. Br c. Na d. K e. N f. Cl 9. In each group, identify the atom with the largest atomic radius a. Sn, Xe, Rb, Sr b. Rn, He, Xe, Kr c. Pb, Ba, Cs, At 10. In each group, identify the atom with the highest ionization energy a. Cs, K, Li b. Ba, Sr, Ca c. I, Br, Cl d. Mg, Si, S 11. Define the following: ionic bond, covalent bond, polar covalent bond, nonpolar covalent bond 12. Define electronegativity and describe the trend across a period and down a group. What type of element has the highest electronegativity? What type has the lowest electronegativity? 13. Identify the type of bond in each: a. K-N b. Cs-O c. C-N d. O-F e. Na-Cl f. Cl-F g. Cl-O h. Ca-Cl 14. Who first arranged the periodic table in order of increasing atomic mass? 15. Draw Lewis structures for the following and predict the molecular shape. a. H2S b. PH3 c. O2 d. sulfate ion e. SO2 16. Calculate the wavelength and energy of 96.6MHz radio waves. f. BF3 17. What is the frequency and energy of electromagnetic radiation having a wavelength of 3.33 X 10 -8m? 18. Predict the ions that the following elements might form: a. F b. K c. I d. Ga e. Fe (trick question) f. P g. Ag