Naming and Writing Formulas for
Molecular Compounds
One milligram of gold is worth
only about one cent, but one
kilogram of gold is worth
approximately $12,500. The
correct prefix ( milli- or kilo-)
makes quite a difference!
Prefixes are important in
chemistry, too. The prefixes
in the name of a binary
molecular compound tell you
its composition.
Naming and Writing
Formulas for Molecular
Compounds
>
Carbon and oxygen combine to form
carbon monoxide (CO) and carbon dioxide
(CO2), but these two invisible gases are
very different.
Slide
2 of 15
© Copyright Pearson Prentice Hall
End Show
Naming and Writing
Formulas for Molecular
Compounds
>
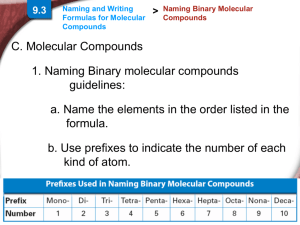
A prefix tells how many atoms of each
element are present in one molecule.
Prefix
Number
Prefix
Number
mono-
1
hexa-
6
di-
2
hepta-
7
tri-
3
octa-
8
tetra-
4
nona-
9
penta-
5
deca-
10
carbon monoxide (CO)
carbon dioxide (CO2)
© Copyright Pearson Prentice Hall
Slide
3 of 15
End Show
Naming and Writing
Formulas for Molecular
Compounds
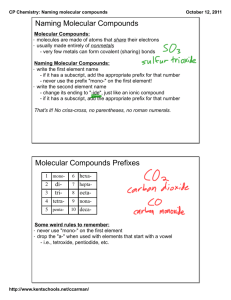
Molecular Naming> Rules:
1) Name the elements in the order
listed in the formula.
2) Use prefixes to show the number
of each atom.
3) The suffix of second element is -ide.
4) No prefix mono- for first element.
CCl4
N 2O
© Copyright Pearson Prentice Hall
SO3
H 2O
Slide
4 of 15
End Show
Naming and Writing
Formulas for Molecular
Compounds
>
Silicon carbide is a hard
material like
diamond. The name silicon carbide has no
prefixes, so the subscripts of silicon and
carbon must be one. Thus, the formula for
silicon carbide is SiC.
Slide
5 of 15
© Copyright Pearson Prentice Hall
End Show
Quick Quiz!
1. Which of the following compounds is named
INCORRECTLY?
A. CS2, carbon disulfide
B. BCl3, boron trichloride
C. IF7, iodine heptafluoride
D. PCl5, phosphorus hexachloride
Slide
6 of 15
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
2. Which of the following molecular compounds
is named INCORRECTLY?
A. AsCl3, arsenic trichloride
B. C2O5, dicarbon pentoxide
C. CF4, carbon tetrafluoride
D. H3As, hydrogen arsenide
Slide
7 of 15
© Copyright Pearson Prentice Hall
End Show
Quick Quiz.
3. The correct formula for
tetraphosphorus trisulfide is
A. P3S4
B. S3P4
C. P4S3
D. S4P3
Slide
8 of 15
© Copyright Pearson Prentice Hall
End Show