9.3
Naming and Writing Formulas for
Molecular Compounds
One milligram of gold is worth
only about one cent, but one
kilogram of gold is worth
approximately $12,500. The
correct prefix ( milli- or kilo-)
makes quite a difference!
Prefixes are important in
chemistry, too. The prefixes
in the name of a binary
molecular compound tell you
its composition.
© Copyright Pearson Prentice Hall
Slide
1 of 15
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
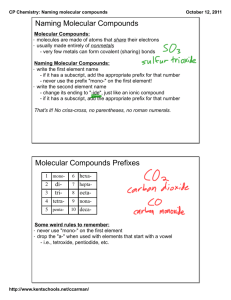
Naming Binary Molecular
Compounds
Carbon and oxygen combine to form carbon
monoxide (CO) and carbon dioxide (CO2), but
these two invisible gases are very different.
Slide
2 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
A prefix in the name of a binary molecular
compound tells how many atoms of an
element are present in each molecule of
the compound.
Slide
3 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
Some guidelines for naming binary
molecular compounds:
• Name the elements in the order listed
in the formula.
• Use prefixes to indicate the number of
each kind of atom.
Slide
4 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Naming Binary Molecular
Compounds
• Omit the prefix mono- when the
formula contains only one atom of the
first element in the name.
• The suffix of the name of the second
element is -ide.
Slide
5 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Writing Formulas for Binary
Molecular Compounds
Use the prefixes in the name to tell you the
subscript of each element in the formula.
Then write the correct symbols for the two
elements with the appropriate subscripts.
Slide
6 of 15
© Copyright Pearson Prentice Hall
9.3
Naming and Writing
Formulas for Molecular
Compounds
>
Writing Formulas for Binary
Molecular Compounds
Silicon carbide is a hard material like diamond.
The name silicon carbide has no prefixes, so the
subscripts of silicon and carbon must be one.
Thus, the formula for silicon carbide is SiC.
Slide
7 of 15
© Copyright Pearson Prentice Hall
9.3 Section Quiz.
1. Which of the following compounds is named
INCORRECTLY?
a. CS2, carbon disulfide
b. BCl3, boron trichloride
c. IF7, iodine heptafluoride
d. PCl5, phosphorus hexachloride
Slide
8 of 15
© Copyright Pearson Prentice Hall
9.3 Section Quiz.
2. Which of the following molecular compounds
is named INCORRECTLY?
a. SbCl3, antimony trichloride
b. C2O5, dicarbon pentoxide
c. CF4, carbon tetrafluoride
d. H3As, hydrogen arsenide
Slide
9 of 15
© Copyright Pearson Prentice Hall
9.3 Section Quiz.
3. The correct formula for tetraphosphorus
trisulfide is
a. P3S4
b. S3P4
c. P4S3
d. S4P3
Slide
10 of 15
© Copyright Pearson Prentice Hall