Naming Type III Binary Compounds: Chemistry Guide
advertisement

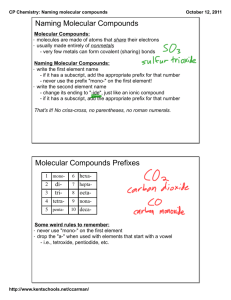
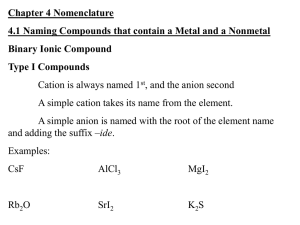
1. What type of elements do type 3 binary compounds contain? a. Nonmetals 2. List the rules for naming type III binary compounds a. The first element in the formula is named first, and the full element name is used b. The second element is named as though it were an anion c. Prefixes are used to denote the numbers of atoms present d. The prefix mono- is never used for naming the first element. Give example 3. List the prefixes and the numbers they indicate from table 4.3 a. 1 Mono-, 2 di-, 3 tri-, 4 tetra-, 5 penta-, 6 hexa-, 7 hepta-, 8 octab. Give students ideas of tricks in memorizing these 4. How do we avoid awkward pronunciation when naming these compounds? a. We drop the final O or A of the prefix when the second element is oxygen b. Avoids double vowel and makes the name less awkward to pronounce c. Have students add ammonia (NH3) to their ion chart 5. List some examples of names of type III binary compounds a. Example 4.4 and 4.5 pg 104-5, Questions #10, 11 pg. 118